Cost-Effectiveness Analysis of Camrelizumab Plus Chemotherapy vs. Chemotherapy Alone as the First-Line Treatment in Patients With IIIB-IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC) Without EGFR and ALK Alteration from a Perspective of Health - Care System in China
- PMID: 35002693
- PMCID: PMC8740086
- DOI: 10.3389/fphar.2021.735536
Cost-Effectiveness Analysis of Camrelizumab Plus Chemotherapy vs. Chemotherapy Alone as the First-Line Treatment in Patients With IIIB-IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC) Without EGFR and ALK Alteration from a Perspective of Health - Care System in China
Abstract
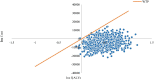
Objective: The CAMEL clinical trial (412 patients were randomly assigned to either camrelizumab plus chemotherapy (n = 205) or chemotherapy alone (n = 207)) demonstrated that camrelizumab plus chemotherapy (CC) improved the overall survival time (OS) and progression-free survival time (PFS) of patients with metastatic nonsquamous non-small cell lung cancer (non-sq NSCLC) without epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations (EGFRm and ALKm) vs. chemotherapy (C) alone. Our objective was to conduct a cost-effectiveness analysis of CC vs. C from a perspective of health - care system in China with a lifetime horizon to identify whether it will be cost-effective. Materials and Methods: A partitioned survival model (PSM) was applied for patients with IIIB-IV non-sq NSCLC without EGFRm and ALKm. Transition parameters and proportions of three health states were derived from the CAMEL trial. The model was designed using a lifetime horizon, a 21-day cycle, and a 5% discount rate of costs and outcomes. It was deemed cost-effective in China if the incremental cost-effectiveness ratio (ICER) value is less than $32,457 per quality adjusted life-year (QALY). Deterministic and probabilistic sensitivity analyses were performed to verify the influence of parameter uncertainty on the results. Results: In the base-case analysis, we found that the ICER of CC compared with C is $-7,382.72/QALY which meant that CC had lower costs and better outcomes. The results of the sensitivity analyses demonstrated that the result was robust for the ICERs never transcending the willingness-to-pay (WTP) threshold. Conclusion: Camrelizumab plus chemotherapy is an obviously cost-effective therapeutic regime for patients of IIIB-IV non-sq NSCLC without EGFRm and ALKm in China at a $32,457 WTP threshold.
Keywords: CAMEL; NSCLC; camrelizumab; cost-effectiveness; nonsquamous non-small cell lung cancer.
Copyright © 2021 Zhu, Xing, Wu, Liang, Han, Lin and Fang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
Cost-effectiveness of camrelizumab plus chemotherapy vs. chemotherapy in the first-line treatment of non-squamous NSCLC: Evidence from China.Front Med (Lausanne). 2023 Feb 14;10:1122731. doi: 10.3389/fmed.2023.1122731. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36865055 Free PMC article.
-
Toripalimab plus chemotherapy vs. chemotherapy in patients with advanced non-small-cell lung cancer: A cost-effectiveness analysis.Front Pharmacol. 2023 Feb 14;14:1131219. doi: 10.3389/fphar.2023.1131219. eCollection 2023. Front Pharmacol. 2023. PMID: 36865925 Free PMC article.
-
Cost-effectiveness analysis of camrelizumab plus chemotherapy as first-line treatment for advanced squamous NSCLC in China.Front Public Health. 2022 Aug 15;10:912921. doi: 10.3389/fpubh.2022.912921. eCollection 2022. Front Public Health. 2022. PMID: 36045725 Free PMC article.
-
Cost-Utility Analysis of Camrelizumab Plus Chemotherapy Versus Chemotherapy Alone as a First-Line Treatment for Advanced Nonsquamous Non-Small Cell Lung Cancer in China.Front Oncol. 2022 Jul 22;12:746526. doi: 10.3389/fonc.2022.746526. eCollection 2022. Front Oncol. 2022. PMID: 35936702 Free PMC article.
-
Economic Evaluation of First-Line Camrelizumab for Advanced Non-small-cell Lung Cancer in China.Front Public Health. 2021 Dec 10;9:743558. doi: 10.3389/fpubh.2021.743558. eCollection 2021. Front Public Health. 2021. PMID: 34957008 Free PMC article.
Cited by
-
Cost-effectiveness of camrelizumab plus chemotherapy vs. chemotherapy in the first-line treatment of non-squamous NSCLC: Evidence from China.Front Med (Lausanne). 2023 Feb 14;10:1122731. doi: 10.3389/fmed.2023.1122731. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36865055 Free PMC article.
-
Toripalimab plus chemotherapy vs. chemotherapy in patients with advanced non-small-cell lung cancer: A cost-effectiveness analysis.Front Pharmacol. 2023 Feb 14;14:1131219. doi: 10.3389/fphar.2023.1131219. eCollection 2023. Front Pharmacol. 2023. PMID: 36865925 Free PMC article.
-
The cost-effectiveness of cemiplimab plus chemotherapy as the first-line treatment for advanced non-small cell lung cancer.Front Pharmacol. 2023 Jul 26;14:1171302. doi: 10.3389/fphar.2023.1171302. eCollection 2023. Front Pharmacol. 2023. PMID: 37564176 Free PMC article.
-
Comparison of camrelizumab, pembrolizumab, tislelizumab, and sintilimab as first-line treatment in patients with non-small cell lung cancer: a retrospective study.J Thorac Dis. 2025 Feb 28;17(2):859-871. doi: 10.21037/jtd-24-1495. Epub 2025 Feb 13. J Thorac Dis. 2025. PMID: 40083528 Free PMC article.
-
Cost‑effectiveness analysis of tislelizumab plus chemotherapy in Chinese patients with advanced or metastatic oesophageal squamous cell carcinoma.Sci Rep. 2024 Jul 31;14(1):17734. doi: 10.1038/s41598-024-68399-3. Sci Rep. 2024. PMID: 39085374 Free PMC article.
References
-
- CSoCOC (2020). Guidelines of Chinese Society of Clinical Oncology (CSCO):Non-Small Cell Lung Cancer (Accessed May 23, 2020).
-
- Drugdataexpy: Marketing Imformation Local Bid-Wining Price. Available From: https://datayaozhcom .
-
- Ferrara R., Imbimbo M., Malouf R., Paget-Bailly S., Calais F., Marchal C., et al. (2020). Single or Combined Immune Checkpoint Inhibitors Compared to First‐line Platinum‐based Chemotherapy with or without Bevacizumab for People with Advanced Non‐small Cell Lung Cancer[J]. Cochrane Database Syst. Rev. 12, CD013257. 10.1002/14651858.CD013257.pub2 - DOI - PMC - PubMed
-
- Fitzmaurice C., Fitzmaurice C., Akinyemiju T. F., Al Lami F. H., Alam T., Alizadeh-Navaei R., et al. (2018). Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 4 (11), 1553–1568. 10.1001/jamaoncol.2018.2706 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

