Translational Pharmacokinetic-Pharmacodynamic Modeling of NaV1.7 Inhibitor MK-2075 to Inform Human Efficacious Dose
- PMID: 35002718
- PMCID: PMC8740778
- DOI: 10.3389/fphar.2021.786078
Translational Pharmacokinetic-Pharmacodynamic Modeling of NaV1.7 Inhibitor MK-2075 to Inform Human Efficacious Dose
Abstract
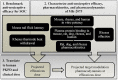
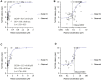
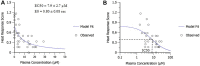
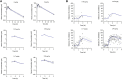
MK-2075 is a small-molecule selective inhibitor of the NaV1.7 channel investigated for the treatment of postoperative pain. A translational strategy was developed for MK-2075 to quantitatively interrelate drug exposure, target modulation, and the desired pharmacological response in preclinical animal models for the purpose of human translation. Analgesics used as a standard of care in postoperative pain were evaluated in preclinical animal models of nociceptive behavior (mouse tail flick latency and rhesus thermode heat withdrawal) to determine the magnitude of pharmacodynamic (PD) response at plasma concentrations associated with efficacy in the clinic. MK-2075 was evaluated in those same animal models to determine the concentration of MK-2075 required to achieve the desired level of response. Translation of MK-2075 efficacious concentrations in preclinical animal models to a clinical PKPD target in humans was achieved by accounting for species differences in plasma protein binding and in vitro potency against the NaV1.7 channel. Estimates of human pharmacokinetic (PK) parameters were obtained from allometric scaling of a PK model from preclinical species and used to predict the dose required to achieve the clinical exposure. MK-2075 exposure-response in a preclinical target modulation assay (rhesus olfaction) was characterized using a computational PKPD model which included a biophase compartment to account for the observed hysteresis. Translation of this model to humans was accomplished by correcting for species differences in PK NaV1.7 potency, and plasma protein binding while assuming that the kinetics of distribution to the target site is the same between humans and rhesus monkeys. This enabled prediction of the level of target modulation anticipated to be achieved over the dosing interval at the projected clinical efficacious human dose. Integration of these efforts into the early development plan informed clinical study design and decision criteria.
Keywords: MK-2075; NaV1.7; PKPD; modeling; nociception; pain.
Copyright © 2021 Ballard, Pall, Vardigan, Zhao, Holahan, Zhou, Jochnowitz, Kraus, Klein, Henze, Houghton, Burgey, Gibson and Struyk.
Conflict of interest statement
The authors JB, PP, JV, FZ, MH, XZ, NJ, RLK, RMK, DH, AH, CB, CG, and AS were employed by the company of Merck and Co., Inc. during the period of this work and may hold stock in those companies.
Figures






References
-
- Ballard J. E., Pall P., Vardigan J., Zhao F., Holahan M. A., Kraus R. (2020). Application of Pharmacokinetic-Pharmacodynamic Modeling to Inform Translation of In Vitro NaV1.7 Inhibition to In Vivo Pharmacological Response in Non-human Primate. Pharm. Res. 37, 181. 10.1007/s11095-020-02914-9 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

