Dysfunctional Vascular Endothelium as a Driver of Atherosclerosis: Emerging Insights Into Pathogenesis and Treatment
- PMID: 35002720
- PMCID: PMC8727904
- DOI: 10.3389/fphar.2021.787541
Dysfunctional Vascular Endothelium as a Driver of Atherosclerosis: Emerging Insights Into Pathogenesis and Treatment
Abstract
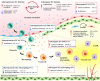
Atherosclerosis, the chronic accumulation of cholesterol-rich plaque within arteries, is associated with a broad spectrum of cardiovascular diseases including myocardial infarction, aortic aneurysm, peripheral vascular disease, and stroke. Atherosclerotic cardiovascular disease remains a leading cause of mortality in high-income countries and recent years have witnessed a notable increase in prevalence within low- and middle-income regions of the world. Considering this prominent and evolving global burden, there is a need to identify the cellular mechanisms that underlie the pathogenesis of atherosclerosis to discover novel therapeutic targets for preventing or mitigating its clinical sequelae. Despite decades of research, we still do not fully understand the complex cell-cell interactions that drive atherosclerosis, but new investigative approaches are rapidly shedding light on these essential mechanisms. The vascular endothelium resides at the interface of systemic circulation and the underlying vessel wall and plays an essential role in governing pathophysiological processes during atherogenesis. In this review, we present emerging evidence that implicates the activated endothelium as a driver of atherosclerosis by directing site-specificity of plaque formation and by promoting plaque development through intracellular processes, which regulate endothelial cell proliferation and turnover, metabolism, permeability, and plasticity. Moreover, we highlight novel mechanisms of intercellular communication by which endothelial cells modulate the activity of key vascular cell populations involved in atherogenesis, and discuss how endothelial cells contribute to resolution biology - a process that is dysregulated in advanced plaques. Finally, we describe important future directions for preclinical atherosclerosis research, including epigenetic and targeted therapies, to limit the progression of atherosclerosis in at-risk or affected patients.
Keywords: atherosclerosis; disturbed flow; endothelial dysfunction; endothelial-to-mesenchymal transition; extracellular vesicles; inflammation resolution; targeted therapy; vascular endothelium.
Copyright © 2021 Botts, Fish and Howe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

