Duclauxin Derivatives From Fungi and Their Biological Activities
- PMID: 35003004
- PMCID: PMC8727740
- DOI: 10.3389/fmicb.2021.766440
Duclauxin Derivatives From Fungi and Their Biological Activities
Abstract
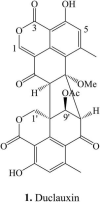
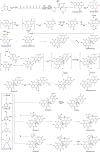
Duclauxin is a heptacyclic oligophenalenone dimer consisting of an isocoumarin and a dihydroisocoumarin unit. These two tricyclic moieties are joined by a cyclopentane ring to form a unique hinge or castanets-like structure. Duclauxin is effective against numerous tumor cell lines because it prevents adenosine triphosphate (ATP) synthesis by inhibiting mitochondrial respiration. There are about 36 reported natural duclauxin analogs mainly produced by 9 Penicillium and Talaromyces species (T. duclauxii, T. aculeatus, T. stipitatus, T. bacillisporus, T. verruculosus, T. macrosporus, P. herquei, P. manginii, and Talaromyces sp.). These metabolites exhibit remarkable biological activities, including antitumor, enzyme inhibition, and antimicrobial, showing tremendous potential in agricultural and medical applications. This review highlights the chemical structures and biological activities of fungal duclauxins, together with biosynthesis, absolute configuration, and mode of action for important duclauxins. Furthermore, phylogenetic analysis and correct names of Penicillium and Talaromyces species producing duclauxins are presented in this review.
Keywords: biological activities; biosynthesis; duclauxin derivatives; fungi; secondary metabolites.
Copyright © 2021 Shahid, Cai, Wang, Zheng, Yang, Mao, Ding and Shan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
New duclauxamide from Penicillium manginii YIM PH30375 and structure revision of the duclauxin family.Org Lett. 2015 Mar 6;17(5):1146-9. doi: 10.1021/acs.orglett.5b00081. Epub 2015 Feb 19. Org Lett. 2015. PMID: 25695664
-
Structurally Diverse Duclauxins from a Coral-Derived Talaromyces sp. and Insight into Determining the Configuration at C-1 of Heptacyclic Duclauxins by 1H NMR.J Nat Prod. 2024 Nov 22;87(11):2592-2603. doi: 10.1021/acs.jnatprod.4c00709. Epub 2024 Nov 4. J Nat Prod. 2024. PMID: 39496137
-
Biosynthesis of Heptacyclic Duclauxins Requires Extensive Redox Modifications of the Phenalenone Aromatic Polyketide.J Am Chem Soc. 2018 Jun 6;140(22):6991-6997. doi: 10.1021/jacs.8b03705. Epub 2018 May 24. J Am Chem Soc. 2018. PMID: 29741874 Free PMC article.
-
Modern taxonomy of biotechnologically important Aspergillus and Penicillium species.Adv Appl Microbiol. 2014;86:199-249. doi: 10.1016/B978-0-12-800262-9.00004-4. Adv Appl Microbiol. 2014. PMID: 24377856 Review.
-
Fungal quinones: diversity, producers, and applications of quinones from Aspergillus, Penicillium, Talaromyces, Fusarium, and Arthrinium.Appl Microbiol Biotechnol. 2021 Nov;105(21-22):8157-8193. doi: 10.1007/s00253-021-11597-0. Epub 2021 Oct 9. Appl Microbiol Biotechnol. 2021. PMID: 34625822 Review.
Cited by
-
High-quality genome assembly and comparative analysis reveal extensive genomic variation in Talaromyces marneffei.Microb Genom. 2025 Apr;11(4):001400. doi: 10.1099/mgen.0.001400. Microb Genom. 2025. PMID: 40294122 Free PMC article.
-
Exploring the Nonenzymatic Origin of Duclauxin-like Natural Products.J Nat Prod. 2024 Sep 27;87(9):2230-2242. doi: 10.1021/acs.jnatprod.4c00558. Epub 2024 Sep 9. J Nat Prod. 2024. PMID: 39252426 Free PMC article.
-
The Outstanding Chemodiversity of Marine-Derived Talaromyces.Biomolecules. 2023 Jun 21;13(7):1021. doi: 10.3390/biom13071021. Biomolecules. 2023. PMID: 37509057 Free PMC article. Review.
-
Alternaria alternata (SDHY01/02), a fungus associated with Lamellodysidea herbacea: its anticancer potential and responsible constituent(s).Int Microbiol. 2023 Nov;26(4):1143-1155. doi: 10.1007/s10123-023-00368-8. Epub 2023 May 5. Int Microbiol. 2023. PMID: 37142818
References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

