Optimized Attenuated Salmonella Typhimurium Suppressed Tumor Growth and Improved Survival in Mice
- PMID: 35003007
- PMCID: PMC8733734
- DOI: 10.3389/fmicb.2021.774490
Optimized Attenuated Salmonella Typhimurium Suppressed Tumor Growth and Improved Survival in Mice
Abstract
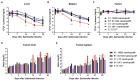
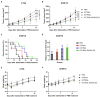
The gram-negative facultative anaerobic bacteria Salmonella enterica serovar Typhimurium (hereafter S. Typhimurium) has always been considered as one candidate of anti-tumor agents or vectors for delivering drug molecules. In this study, we compared several widely studied S. Typhimurium strains in their anti-tumor properties aiming to screen out the best one for further optimization and use in cancer therapy. In terms of the motility, virulence and anti-tumor efficacy, the three strains 14028, SL1344, and UK-1 were similar and obviously better than LT-2, and UK-1 showed the best phenotypes among them. Therefore, the strain UK-1 (D) was selected for the following studies. Its auxotrophic mutant strain (D1) harboring ∆aroA and ∆purM mutations was further optimized through the modification of lipid A structure, generating a new strain named D2 with stronger immunostimulatory activity. Finally, the ∆asd derivative of D2 was utilized as one live vector to deliver anti-tumor molecules including the angiogenesis inhibitor endostatin and apoptosis inducer TRAIL and the therapeutic and toxic-side effects were evaluated in mouse models of colon carcinoma and melanoma. After intraperitoneal infection, engineered Salmonella bacteria equipped with endostatin and/or TRAIL significantly suppressed the tumor growth and prolonged survival of tumor-bearing mice compared to PBS or bacteria carrying the empty plasmid. Consistently, immunohistochemical studies confirmed the colonization of Salmonella bacteria and the expression of anti-tumor molecules inside tumor tissue, which were accompanied by the increase of cell apoptosis and suppression of tumor angiogenesis. These results demonstrated that the beneficial anti-tumor efficacy of attenuated S. Typhimurium bacteria could be improved through delivery of drug molecules with powerful anti-tumor activities.
Keywords: Salmonella Typhimurium; TRAIL; cancer therapy; delivery; endostatin.
Copyright © 2021 Liang, Zhang, Luo, Zhang, Tian, Zhang, Zhang, Ali and Kong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Endostatin gene therapy delivered by attenuated Salmonella typhimurium in murine tumor models.Cancer Gene Ther. 2018 Aug;25(7-8):167-183. doi: 10.1038/s41417-018-0021-6. Epub 2018 May 14. Cancer Gene Ther. 2018. PMID: 29755110
-
aroA-Deficient Salmonella enterica Serovar Typhimurium Is More Than a Metabolically Attenuated Mutant.mBio. 2016 Sep 6;7(5):e01220-16. doi: 10.1128/mBio.01220-16. mBio. 2016. PMID: 27601574 Free PMC article.
-
Attenuated Salmonella Typhimurium with truncated LPS and outer membrane-displayed RGD peptide for cancer therapy.Biomed Pharmacother. 2022 Nov;155:113682. doi: 10.1016/j.biopha.2022.113682. Epub 2022 Sep 12. Biomed Pharmacother. 2022. PMID: 36095964
-
Targeted Cancer Therapy Using Engineered Salmonella typhimurium.Chonnam Med J. 2016 Sep;52(3):173-84. doi: 10.4068/cmj.2016.52.3.173. Epub 2016 Sep 23. Chonnam Med J. 2016. PMID: 27689027 Free PMC article. Review.
-
Genetically engineered Salmonella Typhimurium: Recent advances in cancer therapy.Cancer Lett. 2019 Apr 28;448:168-181. doi: 10.1016/j.canlet.2019.01.037. Epub 2019 Feb 10. Cancer Lett. 2019. PMID: 30753837 Review.
Cited by
-
Salmonella as a Promising Curative Tool against Cancer.Pharmaceutics. 2022 Oct 1;14(10):2100. doi: 10.3390/pharmaceutics14102100. Pharmaceutics. 2022. PMID: 36297535 Free PMC article. Review.
-
Construction and Evaluation of an Efficient Live Attenuated Salmonella Choleraesuis Vaccine and Its Ability as a Vaccine Carrier to Deliver Heterologous Antigens.Vaccines (Basel). 2024 Feb 27;12(3):249. doi: 10.3390/vaccines12030249. Vaccines (Basel). 2024. PMID: 38543883 Free PMC article.
-
Salmonella Typhimurium derived OMV nanoparticle displaying mixed heterologous O-antigens confers immunogenicity and protection against STEC infections in mice.Microb Cell Fact. 2025 Jan 7;24(1):8. doi: 10.1186/s12934-024-02640-6. Microb Cell Fact. 2025. PMID: 39773741 Free PMC article.
-
Engineered oncolytic bacteria HCS1 exerts high immune stimulation and safety profiles for cancer therapy.Theranostics. 2023 Oct 16;13(15):5546-5560. doi: 10.7150/thno.87340. eCollection 2023. Theranostics. 2023. PMID: 37908720 Free PMC article.
-
Bacteria-Based Living Probes: Preparation and the Applications in Bioimaging and Diagnosis.Adv Sci (Weinh). 2024 Jan;11(4):e2306480. doi: 10.1002/advs.202306480. Epub 2023 Nov 30. Adv Sci (Weinh). 2024. PMID: 38032119 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

