Implications of RNA Viruses in the Male Reproductive Tract: An Outlook on SARS-CoV-2
- PMID: 35003013
- PMCID: PMC8739959
- DOI: 10.3389/fmicb.2021.783963
Implications of RNA Viruses in the Male Reproductive Tract: An Outlook on SARS-CoV-2
Abstract
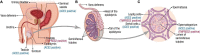
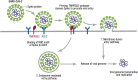
Emerging viral infections continuously pose a threat to human wellbeing. Several RNA viruses have managed to establish access to the male reproductive tract and persist in human semen. The sexual transmission of the virus is of critical public concern. The epidemiological inferences are essential to understand its complexity, particularly the probability of viral transmission from asymptomatic patients or those in the incubation period or from the patient who was previously infected and now fully recovered. From the clinical perspective, negative impacts in the male reproductive tract associated with RNA virus infection have been described, including orchitis, epididymitis, impaired spermatogenesis, and a decrease in sperm quality, which can affect male fertility at different time intervals. The disruption of anatomical barriers due to inflammatory responses might enable the viral invasion into the testis, and the immune privilege status of testes might facilitate a sustained persistence of the virus in the semen. In this review, the current knowledge about other RNA viruses that affect male reproductive health provides the framework to discuss the impact of the SARS-CoV-2 pandemic. The molecular mechanisms, sexual transmission, and viral impacts for mumps, HIV, Zika, and Ebola viruses are explored. We discuss the currently available information on the impact of SARS-CoV-2 and its sequelae in the male reproductive tract, particularly regarding presence in semen, its impact on sexual organs, and sperm quality. To date, no sexual transmission of SARS-CoV-2 has been reported, whereas the identification of viral particles in semen remains conflicting. In the purview of the earlier conducted analyses, it is essential to investigate further the long-term health impacts of SARS-CoV-2 on the male reproductive tract.
Keywords: COVID-19; RNA virus; SARS-CoV-2; male infertility; seminal fluid; sexual transmission.
Copyright © 2021 Zafar, Yu and Li.
Conflict of interest statement
The authors declare that they have no competing or conflict of interests to disclose. The funding bodies are public institutions and had no role in study design, conduct, or interpretation of results and its disposition.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

