Dioctanoyl Ultrashort Tetrabasic β-Peptides Sensitize Multidrug-Resistant Gram-Negative Bacteria to Novobiocin and Rifampicin
- PMID: 35003035
- PMCID: PMC8733726
- DOI: 10.3389/fmicb.2021.803309
Dioctanoyl Ultrashort Tetrabasic β-Peptides Sensitize Multidrug-Resistant Gram-Negative Bacteria to Novobiocin and Rifampicin
Abstract
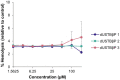
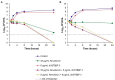
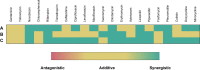
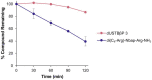
Recently reported peptidomimetics with increased resistance to trypsin were shown to sensitize priority multidrug-resistant (MDR) Gram-negative bacteria to novobiocin and rifampicin. To further optimize proteolytic stability, β-amino acid-containing derivatives of these compounds were prepared, resulting in three dioctanoyl ultrashort tetrabasic β-peptides (dUSTBβPs). The nonhemolytic dUSTBβP 3, comprised of three β3-homoarginine residues and two fatty acyl tails eight carbons long, enhanced the antibacterial activity of various antibiotics from different classes. Notably, compound 3 retained the ability to potentiate novobiocin and rifampicin in wild-type Gram-negative bacteria against MDR clinical isolates of Pseudomonas aeruginosa, Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, and Enterobacter cloacae. dUSTBβP 3 reduced the minimum inhibitory concentration of novobiocin and rifampicin below their interpretative susceptibility breakpoints. Furthermore, compound 3 exhibited improved in vitro stability (86.8 ± 3.7% remaining) relative to its α-amino acid-based counterpart (39.5 ± 7.4% remaining) after a 2 h incubation in human plasma.
Keywords: Acinetobacter baumannii; Escherichia coli; Pseudomonas aeruginosa; antibiotic adjuvant; novobiocin; peptidomimetic; rifampicin; β-amino acid.
Copyright © 2021 Ramirez, Berry, Domalaon, Li, Arthur, Kumar and Schweizer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Effects of Lysine N-ζ-Methylation in Ultrashort Tetrabasic Lipopeptides (UTBLPs) on the Potentiation of Rifampicin, Novobiocin, and Niclosamide in Gram-Negative Bacteria.Antibiotics (Basel). 2022 Mar 3;11(3):335. doi: 10.3390/antibiotics11030335. Antibiotics (Basel). 2022. PMID: 35326798 Free PMC article.
-
Dilipid Ultrashort Tetrabasic Peptidomimetics Potentiate Novobiocin and Rifampicin Against Multidrug-Resistant Gram-Negative Bacteria.ACS Infect Dis. 2020 Jun 12;6(6):1413-1426. doi: 10.1021/acsinfecdis.0c00017. Epub 2020 May 7. ACS Infect Dis. 2020. PMID: 32357292
-
Repurposing Azithromycin and Rifampicin Against Gram-Negative Pathogens by Combination With Peptidomimetics.Front Cell Infect Microbiol. 2019 Jul 2;9:236. doi: 10.3389/fcimb.2019.00236. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31334131 Free PMC article.
-
Antibiotic Potentiation in Multidrug-Resistant Gram-Negative Pathogenic Bacteria by a Synthetic Peptidomimetic.ACS Infect Dis. 2021 Aug 13;7(8):2152-2163. doi: 10.1021/acsinfecdis.1c00147. Epub 2021 Jul 6. ACS Infect Dis. 2021. PMID: 34227804
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
Cited by
-
Antimicrobial Peptide Mimics for Clinical Use: Does Size Matter?Front Immunol. 2022 May 26;13:915368. doi: 10.3389/fimmu.2022.915368. eCollection 2022. Front Immunol. 2022. PMID: 35720375 Free PMC article. Review.
-
Lipopeptide adjuvants for antibiotics and vaccines: the future step in the fight against multidrug-resistant and extensively drug-resistant pathogens.Explor Drug Sci. 2024;2:203-233. doi: 10.37349/eds.2024.00043. Epub 2024 Apr 29. Explor Drug Sci. 2024. PMID: 40842601 Free PMC article.
-
Effects of Lysine N-ζ-Methylation in Ultrashort Tetrabasic Lipopeptides (UTBLPs) on the Potentiation of Rifampicin, Novobiocin, and Niclosamide in Gram-Negative Bacteria.Antibiotics (Basel). 2022 Mar 3;11(3):335. doi: 10.3390/antibiotics11030335. Antibiotics (Basel). 2022. PMID: 35326798 Free PMC article.
References
-
- Andreev K., Bianchi C., Laursen J. S., Citterio L., Hein-Kristensen L., Gram L., et al. . (2014). Guanidino groups greatly enhance the action of antimicrobial peptidomimetics against bacterial cytoplasmic membranes. Biochim. Biophys. Acta Biomembr. 1838, 2492–2502. doi: 10.1016/j.bbamem.2014.05.022, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

