Case Report: First Case of Cefotaxime-Sulbactam-Induced Acute Intravascular Hemolysis in a Newborn With ABO Blood Type Incompatibility by the Mechanism of Non-Immunologic Protein Adsorption
- PMID: 35003054
- PMCID: PMC8727536
- DOI: 10.3389/fimmu.2021.698541
Case Report: First Case of Cefotaxime-Sulbactam-Induced Acute Intravascular Hemolysis in a Newborn With ABO Blood Type Incompatibility by the Mechanism of Non-Immunologic Protein Adsorption
Abstract
Background: ABO blood type incompatibility hemolytic disease of newborn (ABO-HDN) and drug-induced immune hemolytic anemia (DIIHA) due to non-immunologic protein adsorption (NIPA) mainly cause extravascular hemolysis. All the reported severe DIIHA were caused by drug-induced antibodies, and rare report of acute intravascular hemolysis was caused by the NIPA mechanism or ABO-HDN.
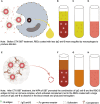
Case presentation: We report the first case of acute intravascular hemolysis induced by cefotaxime sodium - sulbactam sodium (CTX - SBT) in a case of ABO-HDN which resulted in death at 55 h after birth. The mother's blood type was O and RhD-positive, and the newborn's blood type was B and RhD-positive. No irregular red blood cell (RBC) antibodies or drug-dependent antibodies related to CTX or SBT was detected in the mother's plasma and the plasma or the RBC acid eluent of the newborn. Before the newborn received CTX - SBT treatment, the result of direct antiglobulin test (DAT) was negative while anti-B was positive (2 +) in both plasma and acid eluent. After the newborn received CTX - SBT treatment, the results of DAT for anti-IgG and anti-C3d were both positive, while anti-B was not detected in plasma, but stronger anti-B (3 +) was detected in acid eluent. In vitro experiments confirmed that NIPA of SBT promoted the specific binding of maternal-derived IgG anti-B to B antigen on RBCs of the newborn, thereby inducing acute intravascular hemolysis.
Conclusion: The NIPA effect of SBT promoted the specific binding of mother-derived IgG anti-B in newborn's plasma to the newborn's RBC B antigens and formed an immune complex, and then activated complement, which led to acute intravascular hemolysis. Drugs such as SBT with NIPA effect should not be used for newborns with HDN.
Keywords: acute intravascular hemolysis; cefotaxime; drug-induced immune hemolytic anemia (DIIHA); hemolytic disease of newborn (HDN); non-immunologic protein adsorption (NIPA); sulbactam.
Copyright © 2021 Wu, Wu, Yang, Chen, Li, Guo and Xiong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
IgG subclasses of anti-A and anti-B antibodies bound to the cord red cells in ABO incompatible pregnancies.Vox Sang. 1989;56(3):181-6. doi: 10.1111/j.1423-0410.1989.tb02023.x. Vox Sang. 1989. PMID: 2728395
-
[Incidence, laboratory diagnosis and serologic prediction of hemolytic disease of newborn infants due to ABO incompatibility].Padiatr Padol. 1984;19(3):263-78. Padiatr Padol. 1984. PMID: 6472867 German.
-
A study on diagnostic performance of different immunohematological diagnostic tests in assessing the prevalence of ABO Hemolytic Disease of Newborn in the antenatal O group mothers and their neonatal outcome in a tertiary care hospital in Northern India.Transfus Apher Sci. 2024 Feb;63(1):103864. doi: 10.1016/j.transci.2023.103864. Epub 2023 Dec 13. Transfus Apher Sci. 2024. PMID: 38135544
-
ABO hemolytic disease of the newborn: a need for clarity and consistency in diagnosis.J Perinatol. 2023 Feb;43(2):242-247. doi: 10.1038/s41372-022-01556-6. Epub 2022 Nov 8. J Perinatol. 2023. PMID: 36344813 Review.
-
Hemolytic disease of the fetus and newborn with late-onset anemia due to anti-M: a case report and review of the Japanese literature.Transfus Med Rev. 2014 Jan;28(1):1-6. doi: 10.1016/j.tmrv.2013.10.002. Epub 2013 Oct 19. Transfus Med Rev. 2014. PMID: 24262303 Review.
Cited by
-
Microspheres with 2D rGO/Alginate Matrix for Unusual Prolonged Release of Cefotaxime.Nanomaterials (Basel). 2023 May 1;13(9):1527. doi: 10.3390/nano13091527. Nanomaterials (Basel). 2023. PMID: 37177072 Free PMC article.
-
The First-Reported Case of Drug-Induced Hemolytic Anemia by Piperacillin-Tazobactam in a Premature Neonate: A Case Report and Literature Review.Cureus. 2023 Mar 8;15(3):e35915. doi: 10.7759/cureus.35915. eCollection 2023 Mar. Cureus. 2023. PMID: 37038577 Free PMC article.
-
Piperacillin-tazobactam induced immune hemolytic anemia led to increased renal impairment and eventual death from multiple organ failure in a patient with hypertensive nephropathy: case report and literature review.BMC Nephrol. 2023 Jun 14;24(1):173. doi: 10.1186/s12882-023-03235-w. BMC Nephrol. 2023. PMID: 37316798 Free PMC article. Review.
References
-
- Garratty G, Arndt PA. Positive Direct Antiglobulin Tests and Haemolytic Anaemia Following Therapy With Beta-Lactamase Inhibitor Containing Drugs May Be Associated With Non-Immunologic Adsorption of Protein Onto Red Blood Cells. Br J Haematol (1998) 100:777–83. doi: 10.1046/j.1365-2141.1998.00615.x - DOI - PubMed
-
- Garratty G. Arndt, P.A. Drugs That Have Been Shown to Cause Drug-Induced Immune Hemolytic Anemia or Positive Direct Antiglobulin Tests: Some Interesting Findings Since 2007. Immunohematology (2014) 30(2):66–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

