Proteasome α6 Subunit Negatively Regulates the JAK/STAT Pathway and Blood Cell Activation in Drosophila melanogaster
- PMID: 35003057
- PMCID: PMC8727353
- DOI: 10.3389/fimmu.2021.729631
Proteasome α6 Subunit Negatively Regulates the JAK/STAT Pathway and Blood Cell Activation in Drosophila melanogaster
Abstract
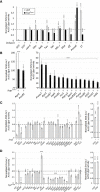
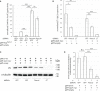
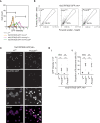
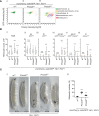
JAK/STAT signaling regulates central biological functions such as development, cell differentiation and immune responses. In Drosophila, misregulated JAK/STAT signaling in blood cells (hemocytes) induces their aberrant activation. Using mass spectrometry to analyze proteins associated with a negative regulator of the JAK/STAT pathway, and by performing a genome-wide RNAi screen, we identified several components of the proteasome complex as negative regulators of JAK/STAT signaling in Drosophila. A selected proteasome component, Prosα6, was studied further. In S2 cells, Prosα6 silencing decreased the amount of the known negative regulator of the pathway, ET, leading to enhanced expression of a JAK/STAT pathway reporter gene. Silencing of Prosα6 in vivo resulted in activation of the JAK/STAT pathway, leading to the formation of lamellocytes, a specific hemocyte type indicative of hemocyte activation. This hemocyte phenotype could be partially rescued by simultaneous knockdown of either the Drosophila STAT transcription factor, or MAPKK in the JNK-pathway. Our results suggest a role for the proteasome complex components in the JAK/STAT pathway in Drosophila blood cells both in vitro and in vivo.
Keywords: Drosophila melanogaster; Eye Transformer; JAK/STAT pathway; RNA interference; fruit fly; hemocyte; lamellocyte; the proteasome complex.
Copyright © 2021 Järvelä-Stölting, Vesala, Maasdorp, Ciantar, Rämet and Valanne.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Eye transformer is a negative regulator of Drosophila JAK/STAT signaling.FASEB J. 2010 Nov;24(11):4467-79. doi: 10.1096/fj.10-162784. Epub 2010 Jul 12. FASEB J. 2010. PMID: 20624926
-
Not4 enhances JAK/STAT pathway-dependent gene expression in Drosophila and in human cells.FASEB J. 2012 Mar;26(3):1239-50. doi: 10.1096/fj.11-195875. Epub 2011 Dec 8. FASEB J. 2012. PMID: 22159038
-
Gα73B is a downstream effector of JAK/STAT signalling and a regulator of Rho1 in Drosophila haematopoiesis.J Cell Sci. 2014 Jan 1;127(Pt 1):101-10. doi: 10.1242/jcs.132852. Epub 2013 Oct 25. J Cell Sci. 2014. PMID: 24163435
-
Organogenesis and tumorigenesis: insight from the JAK/STAT pathway in the Drosophila eye.Dev Dyn. 2010 Oct;239(10):2522-33. doi: 10.1002/dvdy.22394. Dev Dyn. 2010. PMID: 20737505 Free PMC article. Review.
-
Tools and methods for studying the Drosophila JAK/STAT pathway.Methods. 2014 Jun 15;68(1):160-72. doi: 10.1016/j.ymeth.2014.03.023. Epub 2014 Mar 28. Methods. 2014. PMID: 24685392 Review.
Cited by
-
Numerous Serine/Threonine Kinases Affect Blood Cell Homeostasis in Drosophila melanogaster.Cells. 2024 Mar 26;13(7):576. doi: 10.3390/cells13070576. Cells. 2024. PMID: 38607015 Free PMC article.
-
Investigating the Potential Therapeutic Targeting of the JAK-STAT Pathway in Cerebrovascular Diseases: Opportunities and Challenges.Mol Neurobiol. 2025 Jul;62(7):9338-9364. doi: 10.1007/s12035-025-04834-4. Epub 2025 Mar 18. Mol Neurobiol. 2025. PMID: 40102347 Review.
-
A naturally occurring mitochondrial genome variant confers broad protection from infection in Drosophila.PLoS Genet. 2024 Nov 11;20(11):e1011476. doi: 10.1371/journal.pgen.1011476. eCollection 2024 Nov. PLoS Genet. 2024. PMID: 39527645 Free PMC article.
-
Honokiol Prevents Intestinal Barrier Dysfunction in Mice with Severe Acute Pancreatitis and Inhibits JAK/STAT1 Pathway and Acetylation of HMGB1.Chin J Integr Med. 2024 Jun;30(6):534-542. doi: 10.1007/s11655-023-3562-y. Epub 2023 Nov 9. Chin J Integr Med. 2024. PMID: 37943488
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

