Immune Response to Hepatitis B Virus Vaccine Among People Living With HIV: A Meta-Analysis
- PMID: 35003061
- PMCID: PMC8728056
- DOI: 10.3389/fimmu.2021.745541
Immune Response to Hepatitis B Virus Vaccine Among People Living With HIV: A Meta-Analysis
Abstract
Background: There is conflicting evidence about whether a double dose of the hepatitis B virus (HBV) vaccine induces better immunity than the standard-dose vaccine for people living with HIV (PLWH). This study provides a meta-analysis that summarizes the efficacy of HBV vaccine regimens among HIV-infected patients, clarifying the role of particular factors such as dose and frequency of vaccination in vaccine responsiveness and highlighting the need for evidence-based practice to assess HBV vaccination among PLWH.
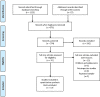
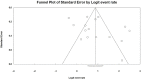
Methods: Randomized clinical trials (RCTs) and prospective studies reporting vaccination response rates among PLWH were found through a search of PubMed, Cochrane, and the Web of Science. The key outcome was vaccine response. A random-effects model was used to estimate the pooled response rate. Subgroup analysis was conducted to evaluate key factors and explore sources of heterogeneity. Possible biases were assessed using quality and publication bias assessment.
Results: Eligible studies included controlled trials that examined the effects of 17 interventional studies with 1,821 participants. Among PLWH who received the HBV vaccine, the pooled response rate of HBV vaccination was 71.5% (95% CI 64.0%-77.9%, p < 0.001). Compared with the standard dose (65.5%, 95% CI 53.1%-76.1%), the double dose (75.2%, 95% CI 66.2%-82.5%) was associated with a better response rate [Q(1) = 19.617, p < 0.001]. When stratified by schedule, the four-dose schedule (89.7%, 95% CI 83.1%-93.9%) had a higher response rate than the three-dose schedule (63.3%, 95% CI 56.6%-69.4%) and the difference was significant [Q(1) = 88.305, p < 0.001]. PLWH with higher CD4+ T-cell counts (>500 cells/mm3) at the time of vaccination had better response rates [Q(1) = 88.305, p < 0.001].
Conclusions: In this meta-analysis, the double dose of the HBV vaccine and multiple injections were associated with better immune responses than the standard HBV vaccine regimen in PLWH. Higher seroconversion rates were observed in PLWH with high CD4+ T-cell levels, indicating that individuals infected with HIV should receive the HBV vaccine as soon as possible after diagnosis.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/.
Keywords: hepatitis B virus; human immunodeficiency virus; immune response; response rate; vaccine.
Copyright © 2021 Tian, Hua, Wu, Zhang, Wang, Wu, Guo and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Sterling RK, Wahed AS, King WC, Kleiner DE, Khalili M, Sulkowski M, et al. . Spectrum of Liver Disease in Hepatitis B Virus (HBV) Patients Co-Infected With Human Immunodeficiency Virus (HIV): Results of the HBV-HIV Cohort Study. Am J Gastroenterol (2019) 114(5):746–57. doi: 10.1038/s41395-018-0409-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

