Intrinsic Strand-Incision Activity of Human UNG: Implications for Nick Generation in Immunoglobulin Gene Diversification
- PMID: 35003074
- PMCID: PMC8730318
- DOI: 10.3389/fimmu.2021.762032
Intrinsic Strand-Incision Activity of Human UNG: Implications for Nick Generation in Immunoglobulin Gene Diversification
Abstract
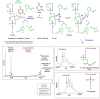
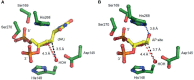
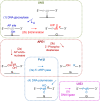
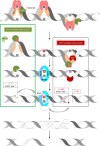
Uracil arises in cellular DNA by cytosine (C) deamination and erroneous replicative incorporation of deoxyuridine monophosphate opposite adenine. The former generates C → thymine transition mutations if uracil is not removed by uracil-DNA glycosylase (UDG) and replaced by C by the base excision repair (BER) pathway. The primary human UDG is hUNG. During immunoglobulin gene diversification in activated B cells, targeted cytosine deamination by activation-induced cytidine deaminase followed by uracil excision by hUNG is important for class switch recombination (CSR) and somatic hypermutation by providing the substrate for DNA double-strand breaks and mutagenesis, respectively. However, considerable uncertainty remains regarding the mechanisms leading to DNA incision following uracil excision: based on the general BER scheme, apurinic/apyrimidinic (AP) endonuclease (APE1 and/or APE2) is believed to generate the strand break by incising the AP site generated by hUNG. We report here that hUNG may incise the DNA backbone subsequent to uracil excision resulting in a 3´-α,β-unsaturated aldehyde designated uracil-DNA incision product (UIP), and a 5´-phosphate. The formation of UIP accords with an elimination (E2) reaction where deprotonation of C2´ occurs via the formation of a C1´ enolate intermediate. UIP is removed from the 3´-end by hAPE1. This shows that the first two steps in uracil BER can be performed by hUNG, which might explain the significant residual CSR activity in cells deficient in APE1 and APE2.
Keywords: class switch recombination; cytosine deamination; human UNG; immunoglobulin diversification; somatic hypermutation.
Copyright © 2021 Alexeeva, Moen, Xu, Rasmussen, Leiros, Kirpekar, Klungland, Alsøe, Nilsen and Bjelland.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. 2nd ed. Washington, DC: ASM Press; (2006).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

