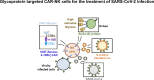
Glycoprotein Targeted CAR-NK Cells for the Treatment of SARS-CoV-2 Infection
- PMID: 35003077
- PMCID: PMC8732772
- DOI: 10.3389/fimmu.2021.763460
Glycoprotein Targeted CAR-NK Cells for the Treatment of SARS-CoV-2 Infection
Abstract
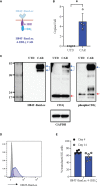
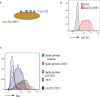
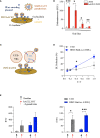
H84T-Banana Lectin (BanLec) CAR-NK cells bind high mannose glycosites that decorate the SARS-CoV-2 envelope, thereby decreasing cellular infection in a model of SARS-CoV-2. H84T-BanLec CAR-NK cells are innate effector cells, activated by virus. This novel cellular agent is a promising therapeutic, capable of clearing circulating SARS-CoV-2 virus and infected cells. Banana Lectin (BanLec) binds high mannose glycans on viral envelopes, exerting an anti-viral effect. A point mutation (H84T) divorces BanLec mitogenicity from antiviral activity. SARS-CoV-2 contains high mannose glycosites in proximity to the receptor binding domain of the envelope Spike (S) protein. We designed a chimeric antigen receptor (CAR) that incorporates H84T-BanLec as the extracellular moiety. Our H84T-BanLec CAR was devised to specifically direct NK cell binding of SARS-CoV-2 envelope glycosites to promote viral clearance. The H84T-BanLec CAR was stably expressed at high density on primary human NK cells during two weeks of ex vivo expansion. H84T-BanLec CAR-NK cells reduced S-protein pseudotyped lentiviral infection of 293T cells expressing ACE2, the receptor for SARS-CoV-2. NK cells were activated to secrete inflammatory cytokines when in culture with virally infected cells. H84T-BanLec CAR-NK cells are a promising cell therapy for further testing against wild-type SARS-CoV-2 virus in models of SARS-CoV-2 infection. They may represent a viable off-the-shelf immunotherapy for patients suffering from COVID-19.
Keywords: Banana Lectin; CAR-NK cells; COVID-19; SARS-CoV-2; glycobiology; immunotherapy.
Copyright © 2021 Christodoulou, Rahnama, Ravich, Seo, Zolov, Marple, Markovitz and Bonifant.
Conflict of interest statement
CB, IC, and DM have pending patent applications describing the use of H84T-BanLec and H84T-BanLec effector cell targeting of SARS-CoV-2. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A molecularly engineered, broad-spectrum anti-coronavirus lectin inhibits SARS-CoV-2 and MERS-CoV infection in vivo.Cell Rep Med. 2022 Oct 18;3(10):100774. doi: 10.1016/j.xcrm.2022.100774. Epub 2022 Sep 29. Cell Rep Med. 2022. PMID: 36195094 Free PMC article.
-
S309-CAR-NK cells bind the Omicron variants in vitro and reduce SARS-CoV-2 viral loads in humanized ACE2-NSG mice.J Virol. 2024 Jun 13;98(6):e0003824. doi: 10.1128/jvi.00038-24. Epub 2024 May 20. J Virol. 2024. PMID: 38767356 Free PMC article.
-
CAR-NK Cells Effectively Target SARS-CoV-2-Spike-Expressing Cell Lines In Vitro.Front Immunol. 2021 Jul 23;12:652223. doi: 10.3389/fimmu.2021.652223. eCollection 2021. Front Immunol. 2021. PMID: 34367128 Free PMC article.
-
CAR-NK cell therapy: a potential antiviral platform.Sci Bull (Beijing). 2025 Mar 15;70(5):765-777. doi: 10.1016/j.scib.2025.01.002. Epub 2025 Jan 3. Sci Bull (Beijing). 2025. PMID: 39837721 Review.
-
The Use of Lectins as Tools to Combat SARS-CoV-2.Curr Pharm Des. 2021;27(41):4212-4222. doi: 10.2174/1381612827666210830094743. Curr Pharm Des. 2021. PMID: 34459375 Review.
Cited by
-
Exploring the Utility of NK Cells in COVID-19.Biomedicines. 2022 Apr 26;10(5):1002. doi: 10.3390/biomedicines10051002. Biomedicines. 2022. PMID: 35625739 Free PMC article. Review.
-
Targeting glycans for CAR therapy: The advent of sweet CARs.Mol Ther. 2022 Sep 7;30(9):2881-2890. doi: 10.1016/j.ymthe.2022.07.006. Epub 2022 Jul 12. Mol Ther. 2022. PMID: 35821636 Free PMC article. Review.
-
Mesothelin-based CAR-T cells exhibit potent antitumor activity against ovarian cancer.J Transl Med. 2024 Apr 18;22(1):367. doi: 10.1186/s12967-024-05174-y. J Transl Med. 2024. PMID: 38637885 Free PMC article.
-
Diverse potential of chimeric antigen receptor-engineered cell therapy: Beyond cancer.Clin Transl Med. 2025 Apr;15(4):e70306. doi: 10.1002/ctm2.70306. Clin Transl Med. 2025. PMID: 40205818 Free PMC article. Review.
-
Gene-Based Natural Killer Cell Therapies for the Treatment of Pediatric Hematologic Malignancies.Hematol Oncol Clin North Am. 2022 Aug;36(4):745-768. doi: 10.1016/j.hoc.2022.03.007. Epub 2022 Jun 27. Hematol Oncol Clin North Am. 2022. PMID: 35773048 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

