Inhibition of HMGB1 Ameliorates the Maternal-Fetal Interface Destruction in Unexplained Recurrent Spontaneous Abortion by Suppressing Pyroptosis Activation
- PMID: 35003098
- PMCID: PMC8732860
- DOI: 10.3389/fimmu.2021.782792
Inhibition of HMGB1 Ameliorates the Maternal-Fetal Interface Destruction in Unexplained Recurrent Spontaneous Abortion by Suppressing Pyroptosis Activation
Abstract
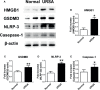
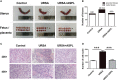
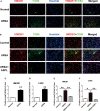
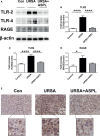
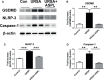
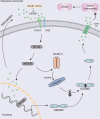
Recurrent spontaneous abortion (RSA) is a common complication of pregnancy that affects the physical and mental health of pregnant women, and approximately 50% of the mechanisms are unclear. Our previous studies have found that high mobility group box 1 (HMGB1) molecules are highly expressed at the maternal-fetal interface of unexplained recurrent spontaneous abortion (URSA) patients. The purpose of this study was to further detect the expression of HMGB1 and pyroptosis in decidual tissue of URSA patients, and explore the potential mechanism of the protective role of HMGB1 in URSA patients and mouse model. The decidua tissues of 75 URSA patients and 75 women who actively terminated pregnancy were collected, and URSA mouse models were established and treated with HMGB1 inhibitor-aspirin. The expression of HMGB1, and their receptors (RAGE, TLR2, TLR4), pyroptosis-associated proteins (NLRP-3, caspase-1, GSDMD) and NF-κB was examined at the maternal-fetal interface of human and mouse. Our study found that HMGB1, NLRP-3, Caspase-1, GSDMD, RAGE, TLR2 and TLR4 were highly expressed and NF-κB signaling pathway were activated in the decidua tissue of URSA group. Moreover, immune cell disorder and co-localization of HMGB1 and macrophages were found at the maternal-fetal interface of URSA mice. However, HMGB1, TLR2, TLR4, NF-κB, and pyroptosis-associated proteins can be down-regulated by administering low-dose aspirin. These data may indicate that highly expressed HMGB1 was actively secreted by macrophages and then activated pyroptosis through the TLR2/TLR4-NF-κB pathway to cause aseptic inflammation, leading to the occurrence and development of URSA. Moreover, low-dose aspirin can reduce HMGB1 protein levels of serum and decidual in URSA.
Keywords: URSA; high mobility group B-1; macrophage; maternal-fetal Interface; pyroptosis.
Copyright © 2021 Zhu, Zou, Liu, Wang, Ma, Yin, Peng, Li, Yang, Ren, Zhang, Zhou, Wang, Cao and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Destruction in maternal-fetal interface of URSA patients via the increase of the HMGB1-RAGE/TLR2/TLR4-NF-κB signaling pathway.Life Sci. 2020 Jun 1;250:117543. doi: 10.1016/j.lfs.2020.117543. Epub 2020 Mar 10. Life Sci. 2020. PMID: 32169518
-
HMGB1 induces unexplained recurrent spontaneous abortion by mediating decidual macrophage autophagy.Int Immunopharmacol. 2025 Feb 6;147:113999. doi: 10.1016/j.intimp.2024.113999. Epub 2025 Jan 8. Int Immunopharmacol. 2025. PMID: 39787761
-
Upregulated HMGB1 levels in maternal-fetal interface of patients with unexplained recurrent spontaneous abortion from different sources.J Matern Fetal Neonatal Med. 2022 Dec;35(25):6542-6549. doi: 10.1080/14767058.2021.1918084. Epub 2021 May 4. J Matern Fetal Neonatal Med. 2022. PMID: 33944653
-
Expatiating the molecular approaches of HMGB1 in diabetes mellitus: Highlighting signalling pathways via RAGE and TLRs.Mol Biol Rep. 2021 Feb;48(2):1869-1881. doi: 10.1007/s11033-020-06130-x. Epub 2021 Jan 21. Mol Biol Rep. 2021. PMID: 33479829 Review.
-
Decidual macrophages in recurrent spontaneous abortion.Front Immunol. 2022 Dec 8;13:994888. doi: 10.3389/fimmu.2022.994888. eCollection 2022. Front Immunol. 2022. PMID: 36569856 Free PMC article.
Cited by
-
YAP-mediated trophoblast dysfunction: the common pathway underlying pregnancy complications.Cell Commun Signal. 2023 Dec 14;21(1):353. doi: 10.1186/s12964-023-01371-2. Cell Commun Signal. 2023. PMID: 38098027 Free PMC article. Review.
-
The role of pyroptosis in the occurrence and development of pregnancy-related diseases.Front Immunol. 2024 Sep 16;15:1400977. doi: 10.3389/fimmu.2024.1400977. eCollection 2024. Front Immunol. 2024. PMID: 39351226 Free PMC article. Review.
-
miR-126-5p protects from URSA via inhibiting Caspase-1-dependent pyroptosis of trophoblast cells.Cell Mol Life Sci. 2025 May 15;82(1):204. doi: 10.1007/s00018-025-05713-w. Cell Mol Life Sci. 2025. PMID: 40372489 Free PMC article.
-
Inhibition of Caspase 1 Reduces Blood Pressure, Cytotoxic NK Cells, and Inflammatory T-Helper 17 Cells in Placental Ischemic Rats.Int J Mol Sci. 2024 Jan 10;25(2):863. doi: 10.3390/ijms25020863. Int J Mol Sci. 2024. PMID: 38255935 Free PMC article.
-
Angiogenin Ameliorates Endometritis by Inhibiting NLRP3 Inflammasome Activation.Animals (Basel). 2025 Jul 8;15(14):2002. doi: 10.3390/ani15142002. Animals (Basel). 2025. PMID: 40723465 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

