Rotavirus Interactions With Host Intestinal Epithelial Cells
- PMID: 35003114
- PMCID: PMC8727603
- DOI: 10.3389/fimmu.2021.793841
Rotavirus Interactions With Host Intestinal Epithelial Cells
Abstract
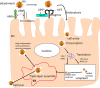
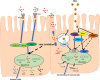
Rotavirus (RV) is the foremost enteric pathogen associated with severe diarrheal illness in young children (<5years) and animals worldwide. RV primarily infects mature enterocytes in the intestinal epithelium causing villus atrophy, enhanced epithelial cell turnover and apoptosis. Intestinal epithelial cells (IECs) being the first physical barrier against RV infection employs a range of innate immune strategies to counteract RVs invasion, including mucus production, toll-like receptor signaling and cytokine/chemokine production. Conversely, RVs have evolved numerous mechanisms to escape/subvert host immunity, seizing translation machinery of the host for effective replication and transmission. RV cell entry process involve penetration through the outer mucus layer, interaction with cell surface molecules and intestinal microbiota before reaching the IECs. For successful cell attachment and entry, RVs use sialic acid, histo-blood group antigens, heat shock cognate protein 70 and cell-surface integrins as attachment factors and/or (co)-receptors. In this review, a comprehensive summary of the existing knowledge of mechanisms underlying RV-IECs interactions, including the role of gut microbiota, during RV infection is presented. Understanding these mechanisms is imperative for developing efficacious strategies to control RV infections, including development of antiviral therapies and vaccines that target specific immune system antagonists within IECs.
Keywords: immune evasion; immune receptors; immunity; intestinal epithelial cells; rotaviruses pathogenesis.
Copyright © 2021 Amimo, Raev, Chepngeno, Mainga, Guo, Saif and Vlasova.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Estes MK, Greenberg HB. Rotaviruses. In: Knipe DM, Howley PM, editors. Fields Virology, 6th ed. Philadelphia: Wolters Kluwer Health /Lippincott Williams & Wilkins; (2013). p. 1347–401.
-
- Engevik MA, Banks LD, Engevik KA, Chang-Graham AL, Perry JL, Hutchinson DS, et al. . Rotavirus Infection Induces Glycan Availability to Promote Ileum-Specific Changes in the Microbiome Aiding Rotavirus Virulence. Gut Microbes (2020) 11(5):1324–47. doi: 10.1080/19490976.2020.1754714 - DOI - PMC - PubMed
-
- Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, Buesa J, et al. . Rotavirus Stimulates Release of Serotonin (5-HT) From Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting. PloS Pathog (2011) 7(7):e1002115. doi: 10.1371/journal.ppat.1002115 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

