Rubella Virus Infected Macrophages and Neutrophils Define Patterns of Granulomatous Inflammation in Inborn and Acquired Errors of Immunity
- PMID: 35003119
- PMCID: PMC8728873
- DOI: 10.3389/fimmu.2021.796065
Rubella Virus Infected Macrophages and Neutrophils Define Patterns of Granulomatous Inflammation in Inborn and Acquired Errors of Immunity
Abstract
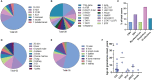
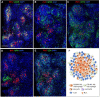
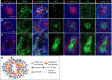
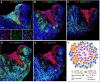
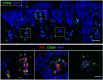
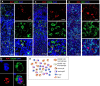
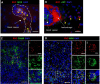
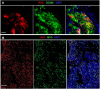
Rubella virus (RuV) has recently been found in association with granulomatous inflammation of the skin and several internal organs in patients with inborn errors of immunity (IEI). The cellular tropism and molecular mechanisms of RuV persistence and pathogenesis in select immunocompromised hosts are not clear. We provide clinical, immunological, virological, and histological data on a cohort of 28 patients with a broad spectrum of IEI and RuV-associated granulomas in skin and nine extracutaneous tissues to further delineate this relationship. Combined immunodeficiency was the most frequent diagnosis (67.8%) among patients. Patients with previously undocumented conditions, i.e., humoral immunodeficiencies, a secondary immunodeficiency, and a defect of innate immunity were identified as being susceptible to RuV-associated granulomas. Hematopoietic cell transplantation was the most successful treatment in this case series resulting in granuloma resolution; steroids, and TNF-α and IL-1R inhibitors were moderately effective. In addition to M2 macrophages, neutrophils were identified by immunohistochemical analysis as a novel cell type infected with RuV. Four patterns of RuV-associated granulomatous inflammation were classified based on the structural organization of granulomas and identity and location of cell types harboring RuV antigen. Identification of conditions that increase susceptibility to RuV-associated granulomas combined with structural characterization of the granulomas may lead to a better understanding of the pathogenesis of RuV-associated granulomas and discover new targets for therapeutic interventions.
Keywords: granuloma treatments; granulomatous inflammation; inborn errors of immunity; macrophages; neutrophils; primary immunodeficiency; skin lesion; vaccine-derived rubella viruses.
Copyright © 2021 Perelygina, Faisthalab, Abernathy, Chen, Hao, Bercovitch, Bayer, Noroski, Lam, Cicalese, Al-Herz, Nanda, Hajjar, Vanden Driessche, Schroven, Leysen, Rosenbach, Peters, Raedler, Albert, Abraham, Rangarjan, Buchbinder, Kobrynski, Pham-Huy, Dhossche, Cunningham Rundles, Meyer, Theos, Atkinson, Musiek, Adeli, Derichs, Walz, Krüger, von Bernuth, Klein, Icenogle, Hauck and Sullivan.
Conflict of interest statement
MA is employed by Sidra Medicine and Hamad Medical Corporation, Qatar. HB is employed by Labor Berlin GmbH, Germany. JH received grants from Immune Deficiency Foundation, the US immunodeficiency network, Chao-physician Scientist award, the Texas Medical Center Digestive Diseases Center and the Jeffrey Modell Foundation. JH received honorarium, consultation fees from Horizon, Pharming, Baxalta, CSL Behring, the National guard, and Al-Faisal University Hospital. TPA received consultation fees from Horizon, Pharming, CSL Behring. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. . Human Inborn Errors of Immunity: 2019 Update on the Classification From the International Union of Immunological Societies Expert Committee. J Clin Immunol (2020) 40(1):24–64. doi: 10.1007/s10875-019-00737-x - DOI - PMC - PubMed
-
- Leung J, Sullivan KE, Perelygina L, Icenogle JP, Fuleihan RL, Lanzieri TM. Prevalence of Granulomas in Patients With Primary Immunodeficiency Disorders, United States: Data From National Health Care Claims and the US Immunodeficiency Network Registry. J Clin Immunol (2018) 38(6):717–26. doi: 10.1007/s10875-018-0534-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

