The Bacterial Microbiome of the Tomato Fruit Is Highly Dependent on the Cultivation Approach and Correlates With Flavor Chemistry
- PMID: 35003161
- PMCID: PMC8740158
- DOI: 10.3389/fpls.2021.775722
The Bacterial Microbiome of the Tomato Fruit Is Highly Dependent on the Cultivation Approach and Correlates With Flavor Chemistry
Abstract
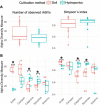
The modes of interactions between plants and plant-associated microbiota are manifold, and secondary metabolites often play a central role in plant-microbe interactions. Abiotic and biotic (including both plant pathogens and endophytes) stress can affect the composition and concentration of secondary plant metabolites, and thus have an influence on chemical compounds that make up for the taste and aroma of fruit. While the role of microbiota in growth and health of plants is widely acknowledged, relatively little is known about the possible effect of microorganisms on the quality of fruit of plants they are colonizing. In this work, tomato (Solanum lycopersicum L.) plants of five different cultivars were grown in soil and in hydroponics to investigate the impact of the cultivation method on the flavor of fruit, and to assess whether variations in their chemical composition are attributable to shifts in bacterial microbiota. Ripe fruit were harvested and used for bacterial community analysis and for the analysis of tomato volatiles, sugars and acids, all contributing to flavor. Fruit grown in soil showed significantly higher sugar content, whereas tomatoes from plants under hydroponic conditions had significantly higher levels of organic acids. In contrast, aroma profiles of fruit were shaped by the tomato cultivars, rather than the cultivation method. In terms of bacterial communities, the cultivation method significantly defined the community composition in all cultivars, with the bacterial communities in hydroponic tomatoes being more variable that those in tomatoes grown in soil. Bacterial indicator species in soil-grown tomatoes correlated with higher concentrations of volatiles described to be perceived as "green" or "pungent." A soil-grown specific reproducibly occurring ASV (amplicon sequence variants) classified as Bacillus detected solely in "Solarino" tomatoes, which were the sweetest among all cultivars, correlated with the amount of aroma-relevant volatiles as well as of fructose and glucose in the fruit. In contrast, indicator bacterial species in hydroponic-derived tomatoes correlated with aroma compounds with "sweet" and "floral" notes and showed negative correlations with glucose concentrations in fruit. Overall, our results point toward a microbiota-related accumulation of flavor and aroma compounds in tomato fruit, which is strongly dependent on the cultivation substrate and approach.
Keywords: aroma and flavor; bacterial microbiota; hydroponics; organoleptic properties; tomato fruit.
Copyright © 2021 Escobar Rodríguez, Novak, Buchholz, Uetz, Bragagna, Gumze, Antonielli and Mitter.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Adalid A. M., Roselló S., Valcárcel M., Nuez F. (2012). Analysis of the genetic control of β-carotene and l-ascorbic acid accumulation in an orange-brownish wild cherry tomato accession. Euphytica 184 251–263. 10.1007/s10681-011-0584-x - DOI
LinkOut - more resources
Full Text Sources

