Effectors of Root-Knot Nematodes: An Arsenal for Successful Parasitism
- PMID: 35003188
- PMCID: PMC8727514
- DOI: 10.3389/fpls.2021.800030
Effectors of Root-Knot Nematodes: An Arsenal for Successful Parasitism
Abstract
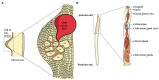
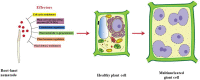
Root-knot nematodes (RKNs) are notorious plant-parasitic nematodes first recorded in 1855 in cucumber plants. They are microscopic, obligate endoparasites that cause severe losses in agriculture and horticulture. They evade plant immunity, hijack the plant cell cycle, and metabolism to modify healthy cells into giant cells (GCs) - RKN feeding sites. RKNs secrete various effector molecules which suppress the plant defence and tamper with plant cellular and molecular biology. These effectors originate mainly from sub-ventral and dorsal oesophageal glands. Recently, a few non-oesophageal gland secreted effectors have been discovered. Effectors are essential for the entry of RKNs in plants, subsequently formation and maintenance of the GCs during the parasitism. In the past two decades, advanced genomic and post-genomic techniques identified many effectors, out of which only a few are well characterized. In this review, we provide molecular and functional details of RKN effectors secreted during parasitism. We list the known effectors and pinpoint their molecular functions. Moreover, we attempt to provide a comprehensive insight into RKN effectors concerning their implications on overall plant and nematode biology. Since effectors are the primary and prime molecular weapons of RKNs to invade the plant, it is imperative to understand their intriguing and complex functions to design counter-strategies against RKN infection.
Keywords: effectors; giant cells; oesophageal glands; plant-nematode interaction; root-knot nematode.
Copyright © 2021 Jagdale, Rao and Giri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Abad P., Williamson V. M. (2010). Plant nematode interaction: A sophisticated dialogue. Adv. Bot. Res. 53, 147–192. doi: 10.1016/S0065-2296(10)53005-2 - DOI
-
- Adam M. A. M., Phillips M. S., Jones J. T., Blok V. C. (2008). Characterisation of the cellulose-binding protein Mj-cbp-1 of the root knot nematode. Meloido. Javanica. Physiol. Mol. Plant Pathol. 72, 21–28. doi: 10.1016/j.pmpp.2008.05.002 - DOI
-
- Adam M. A. M., Phillips M. S., Tzortzakakis E. A., Blok V. C. (2009). Characterisation of mjap genes encoding novel secreted proteins from the root-knot nematode, Meloidogyne javanica. Nematology 11, 253–265. doi: 10.1163/156854109X429583 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

