Classification and Morphology of Rhinocypha spp. (Odonata): A Comprehensive Taxonomic Study Within the Females
- PMID: 35003341
- PMCID: PMC8685346
- DOI: 10.6620/ZS.2021.60-47
Classification and Morphology of Rhinocypha spp. (Odonata): A Comprehensive Taxonomic Study Within the Females
Abstract
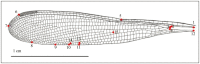
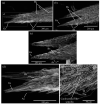
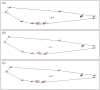
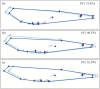
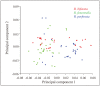
Studies on Odonata have gained attention worldwide as well as locally in Malaysia. Although there is a wealth of data available to be utilized for solving taxonomic problems, ecological and behavioural research areas are more favoured than taxonomy and systematics. Thus, there are confusions over how to correctly identify closely related and sympatric species, especially in female odonates. One such example is in the genus Rhinocypha. Consequently, the present study focuses on taxonomic work, employing multi-approaches in the form of morphological (morphological diagnostics, Field Emission Scanning Electron Microscope (FESEM) and geometric morphometric analysis), applying the molecular technique. Seventeen morphological characteristics were created to differentiate between the females of Rhinocypha spp. A FESEM was used on the female's ovipositor to focus on the anal appendages and sheathing valve (V3). Also, the phylogenetic patterns expressed by COI and 16S rRNA genes, and canonical variate analysis for the wing geometric morphometric revealed three clusters that supported the distinction of the Rhinocypha group. In summary, this study effectively developed an integrated approach of classic morphological and trendy molecular, combined with FESEM microscopy techniques, which provided corroborative evidence and resolved taxonomic uncertainties.
Keywords: 16S rRNA; Dragonflies; Female’s ovipositor; Geometric morphometric; Mitochondrial COI.
Figures










References
-
- Adams DC, Rohlf FJ, Slice D. 2004. Geometric morphometrics: Ten years of progress following the ‘revolution’. Ital J Zool 71:5–16. doi:10.1080/11250000409356545.
-
- Alexander LC, Delion M, Hawthorne DJ, Lamp WO, Funk DH. 2009. Mitochondrial lineages and DNA barcoding of closely related species in the mayfly genus Ephemerella (Ephemeroptera: Ephemerellidae). J North Am Benthol Soc 28:584–595. doi:10.1899/08-150.1.
-
- Andrew CG. 1966. Sexual recognition in adult Erythemis simplicicollis (Odonata: Anisoptera). Ohio J Sci 66:613–617.
-
- Asahina S. 1954. A morphological study of a relict dragonfly Epiophlebia superstes Selys (Odonata, Anisozygoptera). Society for the Promotion of Science. Tokyo, Japan.
-
- Balke M, Wewalka G, Alarie, Ribera I. 2007. Molecular phylogeny of Pacific Island Colymbetinae: Radiation of New Caledonian and Fijian species (Coleoptera, Dytiscidae). Zool Scr 36:173–200. doi:10.1111/j.1463-6409.2006.00265.x.
LinkOut - more resources
Full Text Sources
Miscellaneous
