DABCO-promoted photocatalytic C-H functionalization of aldehydes
- PMID: 35003372
- PMCID: PMC8712972
- DOI: 10.3762/bjoc.17.205
DABCO-promoted photocatalytic C-H functionalization of aldehydes
Abstract
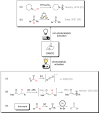
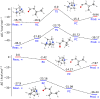
Herein we present a direct application of DABCO, an inexpensive and broadly accessible organic base, as a hydrogen atom transfer (HAT) abstractor in a photocatalytic strategy for aldehyde C-H activation. The acyl radicals generated in this step were arylated with aryl bromides through a well stablished nickel cross-coupling methodology, leading to a variety of interesting aryl ketones in good yields. We also performed computational calculations to shine light in the HAT step energetics and determined an optimized geometry for the transition state, showing that the hydrogen atom transfer between aldehydes and DABCO is a mildly endergonic, yet sufficiently fast step. The same calculations were performed with quinuclidine, for comparison of both catalysts and the differences are discussed.
Keywords: C–H functionalization; DABCO; HAT; photocatalysis.
Copyright © 2021, Maia da Silva Santos et al.
Figures





Similar articles
-
Synthetic and Mechanistic Implications of Chlorine Photoelimination in Nickel/Photoredox C(sp3)-H Cross-Coupling.Acc Chem Res. 2021 Feb 16;54(4):988-1000. doi: 10.1021/acs.accounts.0c00694. Epub 2021 Jan 29. Acc Chem Res. 2021. PMID: 33511841 Free PMC article.
-
Photocatalytic C-H Activation with Alcohol as a Hydrogen Atom Transfer Agent in a 9-Fluorenone Based Metal-Organic Framework.ACS Appl Mater Interfaces. 2021 Jun 9;13(22):25898-25905. doi: 10.1021/acsami.1c03098. Epub 2021 May 27. ACS Appl Mater Interfaces. 2021. PMID: 34043310
-
C-H Alkylation of Aldehydes by Merging TBADT Hydrogen Atom Transfer with Nickel Catalysis.Org Lett. 2021 Jul 16;23(14):5389-5393. doi: 10.1021/acs.orglett.1c01716. Epub 2021 Jun 25. Org Lett. 2021. PMID: 34170145
-
Direct C(sp3)-H functionalization with aryl and alkyl radicals as intermolecular hydrogen atom transfer (HAT) agents.Chem Commun (Camb). 2024 Oct 8;60(81):11450-11465. doi: 10.1039/d4cc03383c. Chem Commun (Camb). 2024. PMID: 39268687 Review.
-
Hydrogen Atom Transfer (HAT): A Versatile Strategy for Substrate Activation in Photocatalyzed Organic Synthesis.European J Org Chem. 2017 Apr 18;2017(15):2056-2071. doi: 10.1002/ejoc.201601485. Epub 2017 Mar 2. European J Org Chem. 2017. PMID: 30147436 Free PMC article. Review.
Cited by
-
Redox-active molecules as organocatalysts for selective oxidative transformations - an unperceived organocatalysis field.Beilstein J Org Chem. 2022 Dec 9;18:1672-1695. doi: 10.3762/bjoc.18.179. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 36570566 Free PMC article.
References
-
- de Azambuja F, Correia C R D. Quim Nova. 2011;34(10):1779–1790. doi: 10.1590/s0100-40422011001000011. - DOI
LinkOut - more resources
Full Text Sources
