Understanding and utilizing textile-based electrostatic flocking for biomedical applications
- PMID: 35003482
- PMCID: PMC8715800
- DOI: 10.1063/5.0070658
Understanding and utilizing textile-based electrostatic flocking for biomedical applications
Abstract
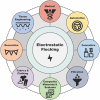
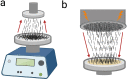
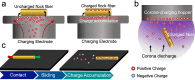
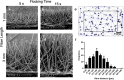
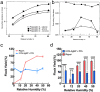
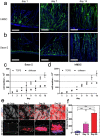
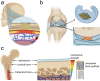
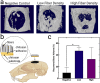
Electrostatic flocking immobilizes electrical charges to the surface of microfibers from a high voltage-connected electrode and utilizes Coulombic forces to propel microfibers toward an adhesive-coated substrate, leaving a forest of aligned fibers. This traditional textile engineering technique has been used to modify surfaces or to create standalone anisotropic structures. Notably, a small body of evidence validating the use of electrostatic flocking for biomedical applications has emerged over the past several years. Noting the growing interest in utilizing electrostatic flocking in biomedical research, we aim to provide an overview of electrostatic flocking, including the principle, setups, and general and biomedical considerations, and propose a variety of biomedical applications. We begin with an introduction to the development and general applications of electrostatic flocking. Additionally, we introduce and review some of the flocking physics and mathematical considerations. We then discuss how to select, synthesize, and tune the main components (flocking fibers, adhesives, substrates) of electrostatic flocking for biomedical applications. After reviewing the considerations necessary for applying flocking toward biomedical research, we introduce a variety of proposed use cases including bone and skin tissue engineering, wound healing and wound management, and specimen swabbing. Finally, we presented the industrial comments followed by conclusions and future directions. We hope this review article inspires a broad audience of biomedical, material, and physics researchers to apply electrostatic flocking technology to solve a variety of biomedical and materials science problems.
© 2021 Author(s).
Figures





References
-
- Kim Y. K., Specialist Yarn and Fabric Structures ( Elsevier, 2011), pp. 287–317.
-
- King W. H., U.S. Patent Office (29 May 1945).
-
- Vellayappan M. V., Jaganathan S. K., and Supriyanto E., RSC Adv. 5, 73225–73240 (2015).10.1039/C5RA11937E - DOI
-
- Gokarneshan N., Curr. Trends Fashion Technol. Text. Eng. 4, 555630 (2018).10.19080/CTFTTE.2018.04.555630 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
