SKI-G-801, an AXL kinase inhibitor, blocks metastasis through inducing anti-tumor immune responses and potentiates anti-PD-1 therapy in mouse cancer models
- PMID: 35003748
- PMCID: PMC8716998
- DOI: 10.1002/cti2.1364
SKI-G-801, an AXL kinase inhibitor, blocks metastasis through inducing anti-tumor immune responses and potentiates anti-PD-1 therapy in mouse cancer models
Abstract
Objectives: AXL-mediated activation of aberrant tyrosine kinase drives various oncogenic processes and facilitates an immunosuppressive microenvironment. We evaluated the anti-tumor and anti-metastatic activities of SKI-G-801, a small-molecule inhibitor of AXL, alone and in combination with anti-PD-1 therapy.
Methods: In vitro pAXL inhibition by SKI-G-801 was performed in both human and mouse cancer cell lines. Immunocompetent mouse models of tumor were established to measure anti-metastatic potential of SKI-G-801. Furthermore, SKI-G-801, anti-PD-1 or their combination was administered as an adjuvant or neoadjuvant in the 4T1 tumor model to assess their potential for clinical application.
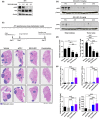
Results: SKI-G-801 robustly inhibited pAXL expression in various cell lines. SKI-G-801 alone or in combination with anti-PD-1 potently inhibited metastasis in B16F10 melanoma, CT26 colon and 4T1 breast models. SKI-G-801 inhibited the growth of B16F10 and 4T1 tumor-bearing mice but not immune-deficient mice. An antibody depletion assay revealed that CD8+ T cells significantly contributed to SKI-G-801-mediated survival. Anti-PD-1 and combination group were observed the increased CD8+Ki67+ and effector T cells and M1 macrophage and decreased M2 macrophage, and granulocytic myeloid-derived suppressor cell (G-MDSC) compared to the control group. The neoadjuvant combination of SKI-G-801 and anti-PD-1 therapy achieved superior survival benefits by inducing more profound T-cell responses in the 4T1 syngeneic mouse model.
Conclusion: SKI-G-801 significantly suppressed tumor metastasis and growth by enhancing anti-tumor immune responses. Our results suggest that SKI-G-801 has the potential to overcome anti-PD-1 therapy resistance and allow more patients to benefit from anti-PD-1 therapy.
Keywords: AXL; immunotherapy; kinase inhibitor; metastasis; small molecule.
© 2022 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

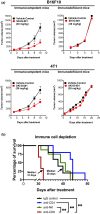
 ). Significance was determined using the log‐rank test; *P ≤ 0.05. (b) Overall survival rate was monitored in SKI‐G‐801‐treated 4T1 tumor‐bearing mice following injection of anti‐CD8, anti‐NK and anti‐CD4 antibodies, and IgG control (n = 5 per group, derived from one independent experiment). Survival was determined by either death or high tumor volume (= 2500 mm3). Significance was determined by the log‐rank test; **P ≤ 0.01.
). Significance was determined using the log‐rank test; *P ≤ 0.05. (b) Overall survival rate was monitored in SKI‐G‐801‐treated 4T1 tumor‐bearing mice following injection of anti‐CD8, anti‐NK and anti‐CD4 antibodies, and IgG control (n = 5 per group, derived from one independent experiment). Survival was determined by either death or high tumor volume (= 2500 mm3). Significance was determined by the log‐rank test; **P ≤ 0.01.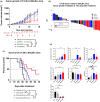
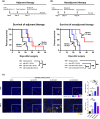
 ), CD3+ cells are marked in pink (
), CD3+ cells are marked in pink ( ), and PD‐L1+ cells are marked in yellow (
), and PD‐L1+ cells are marked in yellow ( ). IHC was done once and CD3+ cells (per mm2), and percentage of PD‐L1+ cells (%) were calculated (right). Values are indicated as means ± SEM, and P‐values were calculated using the Student’s t‐test; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
). IHC was done once and CD3+ cells (per mm2), and percentage of PD‐L1+ cells (%) were calculated (right). Values are indicated as means ± SEM, and P‐values were calculated using the Student’s t‐test; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.References
-
- Lee HJ, Jeng YM, Chen YL, Chung L, Yuan RH. Gas6/Axl pathway promotes tumor invasion through the transcriptional activation of Slug in hepatocellular carcinoma. Carcinogenesis 2014; 35: 769–775. - PubMed
-
- Hasanbasic I, Cuerquis J, Varnum B, Blostein MD. Intracellular signaling pathways involved in Gas6‐Axl‐mediated survival of endothelial cells. Am J Physiol Heart Circ Physiol 2004; 287: H1207–H1213. - PubMed
-
- Lee WP, Wen Y, Varnum B, Hung MC. Akt is required for Axl‐Gas6 signaling to protect cells from E1A‐mediated apoptosis. Oncogene 2002; 21: 329–336. - PubMed
-
- Collett G, Wood A, Alexander MY et al. Receptor tyrosine kinase Axl modulates the osteogenic differentiation of pericytes. Circ Res 2003; 92: 1123–1129. - PubMed
-
- Choi YJ, Kim JH, Rho JK et al. AXL and MET receptor tyrosine kinases are essential for lung cancer metastasis. Oncol Rep 2017; 37: 2201–2208. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
