Maintained partial protection against Streptococcus pneumoniae despite B-cell depletion in mice vaccinated with a pneumococcal glycoconjugate vaccine
- PMID: 35003749
- PMCID: PMC8715227
- DOI: 10.1002/cti2.1366
Maintained partial protection against Streptococcus pneumoniae despite B-cell depletion in mice vaccinated with a pneumococcal glycoconjugate vaccine
Abstract
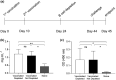
Objectives: Anti-CD20 monoclonal antibody therapy rapidly depletes > 95% of CD20+ B cells from the circulation. B-cell depletion is an effective treatment for autoimmune disease and B-cell malignancies but also increases the risk of respiratory tract infections. This effect on adaptive immunity could be countered by vaccination. We have used mouse models to investigate the effects of B-cell depletion on pneumococcal vaccination, including protection against infection and timing of vaccination in relation to B-cell depletion.
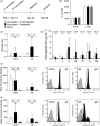
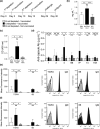
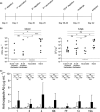
Methods: C57BL/6 female mice were B-cell depleted using anti-CD20 antibody and immunized with two doses of Prevnar-13 vaccine either before or after anti-CD20 treatment. B-cell repertoire and Streptococcus pneumoniae-specific IgG levels were measured using whole-cell ELISA and flow cytometry antibody-binding assay. Protection induced by vaccination was assessed by challenging the mice using a S. pneumoniae pneumonia model.
Results: Antibody responses to S. pneumoniae were largely preserved in mice B-cell depleted after vaccination resulting in full protection against pneumococcal infections. In contrast, mice vaccinated with Prevnar-13 while B cells were depleted (with > 90% reduction in B-cell numbers) had decreased circulating anti-S. pneumoniae IgG and IgM levels (measured using ELISA and flow cytometry antibody binding assays). However, some antibody responses were maintained, and, although vaccine-induced protection against S. pneumoniae infection was impaired, septicaemia was still prevented in 50% of challenged mice.
Conclusions: This study showed that although vaccine efficacy during periods of profound B-cell depletion was impaired some protective efficacy was preserved, suggesting that vaccination remains beneficial.
Keywords: B‐cell depletion; CD20; Streptococcus pneumoniae; immunity; vaccination.
© 2021 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
GW was employed by the company Novartis Institute for BioMedical Research. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The Influence of B Cell Depletion Therapy on Naturally Acquired Immunity to Streptococcus pneumoniae.Front Immunol. 2021 Jan 28;11:611661. doi: 10.3389/fimmu.2020.611661. eCollection 2020. Front Immunol. 2021. PMID: 33584691 Free PMC article.
-
Pneumococcal Conjugate Vaccine Does Not Induce Humoral Response When Administrated Within the Six Months After CD19 CAR T-Cell Therapy.Transplant Cell Ther. 2023 Apr;29(4):277.e1-277.e9. doi: 10.1016/j.jtct.2022.08.011. Epub 2022 Aug 12. Transplant Cell Ther. 2023. PMID: 35970303
-
SLy2-deficiency promotes B-1 cell immunity and triggers enhanced production of IgM and IgG2 antibodies against pneumococcal vaccine.Immun Inflamm Dis. 2020 Dec;8(4):736-752. doi: 10.1002/iid3.365. Epub 2020 Oct 24. Immun Inflamm Dis. 2020. PMID: 33098380 Free PMC article.
-
Vaccination With the Commensal Streptococcus mitis Expressing Pneumococcal Serotype 5 Capsule Elicits IgG/IgA and Th17 Responses Against Streptococcus pneumoniae.Front Immunol. 2021 Apr 19;12:676488. doi: 10.3389/fimmu.2021.676488. eCollection 2021. Front Immunol. 2021. PMID: 33953733 Free PMC article.
-
Mechanisms of Naturally Acquired Immunity to Streptococcus pneumoniae.Front Immunol. 2019 Mar 1;10:358. doi: 10.3389/fimmu.2019.00358. eCollection 2019. Front Immunol. 2019. PMID: 30881363 Free PMC article. Review.
Cited by
-
Technologies and therapeutics for ongoing prevention of respiratory infections.Clin Transl Immunology. 2023 Feb 27;12(2):e1442. doi: 10.1002/cti2.1442. eCollection 2023. Clin Transl Immunology. 2023. PMID: 36861031 Free PMC article. No abstract available.
References
Grants and funding
LinkOut - more resources
Full Text Sources
