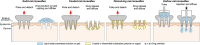
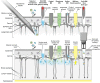
Microneedle systems for delivering nucleic acid drugs
- PMID: 35003824
- PMCID: PMC8726529
- DOI: 10.1007/s40005-021-00558-4
Microneedle systems for delivering nucleic acid drugs
Abstract
Background: Nucleic acid-based gene therapy is a promising technology that has been used in various applications such as novel vaccination platforms for infectious/cancer diseases and cellular reprogramming because of its fast, specific, and effective properties. Despite its potential, the parenteral nucleic acid drug formulation exhibits instability and low efficacy due to various barriers, such as stability concerns related to its liquid state formulation, skin barriers, and endogenous nuclease degradation. As promising alternatives, many attempts have been made to perform nucleic acid delivery using a microneedle system. With its minimal invasiveness, microneedle can deliver nucleic acid drugs with enhanced efficacy and improved stability.
Area covered: This review describes nucleic acid medicines' current state and features and their delivery systems utilizing non-viral vectors and physical delivery systems. In addition, different types of microneedle delivery systems and their properties are briefly reviewed. Furthermore, recent advances of microneedle-based nucleic acid drugs, including featured vaccination applications, are described.
Expert opinion: Nucleic acid drugs have shown significant potential beyond the limitation of conventional small molecules, and the current COVID-19 pandemic highlights the importance of nucleic acid therapies as a novel vaccination platform. Microneedle-mediated nucleic acid drug delivery is a potential platform for less invasive nucleic acid drug delivery. Microneedle system can show enhanced efficacy, stability, and improved patient convenience through self-administration with less pain.
Keywords: Microneedle; Nucleic acid; Vaccination; mRNA; siRNA.
© The Author(s) under exclusive licence to The Korean Society of Pharmaceutical Sciences and Technology 2022.
Conflict of interest statement
Conflict of interestThe authors (I. Noh, K. Lee, and Y.S. Rhee) declare that they have no conflict of interest.
Figures
Similar articles
-
Microneedle Patches as Drug and Vaccine Delivery Platform.Curr Med Chem. 2017;24(22):2413-2422. doi: 10.2174/0929867324666170526124053. Curr Med Chem. 2017. PMID: 28552053 Review.
-
Microneedle Systems for Vaccine Delivery: the story so far.Expert Rev Vaccines. 2020 Dec;19(12):1153-1166. doi: 10.1080/14760584.2020.1874928. Epub 2021 Jan 22. Expert Rev Vaccines. 2020. PMID: 33427523 Review.
-
Efficient delivery of nucleic acid molecules into skin by combined use of microneedle roller and flexible interdigitated electroporation array.Theranostics. 2018 Mar 22;8(9):2361-2376. doi: 10.7150/thno.23438. eCollection 2018. Theranostics. 2018. PMID: 29721085 Free PMC article.
-
Microneedle-mediated delivery of viral vectored vaccines.Expert Opin Drug Deliv. 2017 Oct;14(10):1177-1187. doi: 10.1080/17425247.2017.1230096. Epub 2016 Sep 7. Expert Opin Drug Deliv. 2017. PMID: 27591122 Review.
-
Recent advances in microneedle-based drug delivery: Special emphasis on its use in paediatric population.Eur J Pharm Biopharm. 2019 Mar;136:48-69. doi: 10.1016/j.ejpb.2019.01.005. Epub 2019 Jan 8. Eur J Pharm Biopharm. 2019. PMID: 30633972 Review.
Cited by
-
Biodegradable polymeric insulin microneedles - a design and materials perspective review.Drug Deliv. 2024 Dec;31(1):2296350. doi: 10.1080/10717544.2023.2296350. Epub 2023 Dec 26. Drug Deliv. 2024. PMID: 38147499 Free PMC article. Review.
-
Non-Viral Delivery of Gene Therapy to the Tendon.Polymers (Basel). 2022 Aug 16;14(16):3338. doi: 10.3390/polym14163338. Polymers (Basel). 2022. PMID: 36015594 Free PMC article. Review.
-
Nanomaterials in tuberculosis DNA vaccine delivery: historical perspective and current landscape.Drug Deliv. 2022 Dec;29(1):2912-2924. doi: 10.1080/10717544.2022.2120565. Drug Deliv. 2022. PMID: 36081335 Free PMC article. Review.
-
A microneedle vaccine printer for thermostable COVID-19 mRNA vaccines.Nat Biotechnol. 2024 Mar;42(3):510-517. doi: 10.1038/s41587-023-01774-z. Epub 2023 Apr 24. Nat Biotechnol. 2024. PMID: 37095347 Free PMC article.
-
Looking to the Future of Viral Vectors in Ocular Gene Therapy: Clinical Review.Biomedicines. 2025 Feb 5;13(2):365. doi: 10.3390/biomedicines13020365. Biomedicines. 2025. PMID: 40002778 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials