Extraction of phase-based optoretinograms (ORG) from serial B-scans acquired over tens of seconds by mouse retinal raster scanning OCT system
- PMID: 35003871
- PMCID: PMC8713677
- DOI: 10.1364/BOE.439900
Extraction of phase-based optoretinograms (ORG) from serial B-scans acquired over tens of seconds by mouse retinal raster scanning OCT system
Abstract
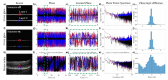
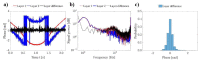
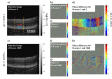
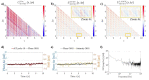
Several specialized retinal optical coherence tomography (OCT) acquisition and processing methods have been recently developed to allow in vivo probing of light-evoked photoreceptors function, focusing on measurements in individual photoreceptors (rods and cones). Recent OCT investigations in humans and experimental animals have shown that the outer segments in dark-adapted rods and cones elongate in response to the visible optical stimuli that bleach fractions of their visual photopigment. We have previously successfully contributed to these developments by implementing OCT intensity-based "optoretinograms" (ORG), the paradigm of using near-infrared OCT (NIR OCT) to measure bleaching-induced back-scattering and/or elongation changes of photoreceptors in the eye in vivo. In parallel, several groups have successfully implemented phase-based ORGs, mainly in human studies, exploiting changes in the phases of back-scattered light. This allowed more sensitive observations of tiny alterations of photoreceptors structures. Applications of the phase-based ORG have been implemented primarily in high speed and cellular resolution AO-OCT systems that can visualize photoreceptor mosaic, allowing phase measurements of path length changes in outer segments of individual photoreceptors. The phase-based ORG in standard resolution OCT systems is much more demanding to implement and has not been explored extensively. This manuscript describes our efforts to implement a phase analysis framework to retinal images acquired with a standard resolution and raster scanning OCT system, which offers much lower phase stability than line-field or full-field OCT detection schemes due to the relatively slower acquisition speed. Our initial results showcase the successful extraction of phase-based ORG signal from the B-scans acquired at ∼100 Hz rate and its favorable comparison with intensity-based ORG signal extracted from the same data sets. We implemented the calculation of phase-based ORG signals using Knox-Thompson paths and modified signal recovery by adding decorrelation weights. The phase-sensitive ORG signal analysis developed here for mouse retinal raster scanning OCT systems could be in principle extended to clinical retinal raster scanning OCT systems, potentially opening doors for clinically friendly ORG probing.
© 2021 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures



Similar articles
-
Polarization optical coherence tomography optoretinography: verifying light-induced photoreceptor outer segment shrinkage and subretinal space expansion.Neurophotonics. 2025 Jan;12(1):015005. doi: 10.1117/1.NPh.12.1.015005. Epub 2025 Jan 27. Neurophotonics. 2025. PMID: 39872019 Free PMC article.
-
Light-adapted flicker optoretinograms captured with a spatio-temporal optical coherence-tomography (STOC-T) system.Biomed Opt Express. 2022 Mar 17;13(4):2186-2201. doi: 10.1364/BOE.444567. eCollection 2022 Apr 1. Biomed Opt Express. 2022. PMID: 35519256 Free PMC article.
-
Functional retinal imaging using adaptive optics swept-source OCT at 1.6 MHz.Optica. 2019 Mar 20;6(3):300-303. doi: 10.1364/OPTICA.6.000300. Optica. 2019. PMID: 33511257 Free PMC article.
-
Toward a clinical optoretinogram: a review of noninvasive, optical tests of retinal neural function.Ann Transl Med. 2021 Aug;9(15):1270. doi: 10.21037/atm-20-6440. Ann Transl Med. 2021. PMID: 34532407 Free PMC article. Review.
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
Cited by
-
The Impact of Photopigment Bleaching on the Human Rod Photoreceptor Subretinal Space Measured Via Optical Coherence Tomography.Invest Ophthalmol Vis Sci. 2024 Mar 5;65(3):20. doi: 10.1167/iovs.65.3.20. Invest Ophthalmol Vis Sci. 2024. PMID: 38470325 Free PMC article.
-
Light-adapted flicker-optoretinography based on raster-scan optical coherence tomography towards clinical translation.Biomed Opt Express. 2024 Sep 26;15(10):6036-6051. doi: 10.1364/BOE.538481. eCollection 2024 Oct 1. Biomed Opt Express. 2024. PMID: 39421778 Free PMC article.
-
Fast and slow light-induced changes in murine outer retina optical coherence tomography: complementary high spatial resolution functional biomarkers.PNAS Nexus. 2022 Oct 14;1(4):pgac208. doi: 10.1093/pnasnexus/pgac208. eCollection 2022 Sep. PNAS Nexus. 2022. PMID: 36338188 Free PMC article.
-
In vivo identification of the retinal layer containing photopigments in OCT images through correlation with two-photon psychophysics.Sci Rep. 2024 Jul 4;14(1):15459. doi: 10.1038/s41598-024-65234-7. Sci Rep. 2024. PMID: 38965299 Free PMC article.
-
On-axis full-field swept-source optical coherence tomography for murine retinal imaging.Opt Lett. 2024 Aug 15;49(16):4630-4633. doi: 10.1364/OL.531116. Opt Lett. 2024. PMID: 39146121 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
