ANXA9 as a novel prognostic biomarker associated with immune infiltrates in gastric cancer
- PMID: 35003923
- PMCID: PMC8684324
- DOI: 10.7717/peerj.12605
ANXA9 as a novel prognostic biomarker associated with immune infiltrates in gastric cancer
Abstract
Background: Gastric cancer (GC) is the most prevalent malignancy among the digestive system tumors. Increasing evidence has revealed that lower mRNA expression of ANXA9 is associated with a poor prognosis in colorectal cancer. However, the role of ANXA9 in GC remains largely unknown.
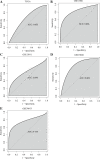
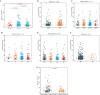
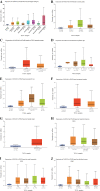
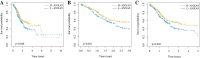
Material and methods: The Gene Expression Profiling Interactive Analysis (GEPIA) and Human Protein Atlas databases were used to investigate the expression of ANXA9 in GC, which was then validated in the four Gene Expression Omnibus (GEO) datasets. The diagnostic value of ANXA9 for GC patients was demonstrated using a receiver operating characteristic (ROC) curve. The correlation between ANXA9 expression and clinicopathological parameters was analyzed in The Cancer Genome Atlas (TCGA) and UALCAN databases. The Kaplan-Meier (K-M) survival curve was used to elucidate the relationship between ANXA9 expression and the survival time of GC patients. We then performed a gene set enrichment analysis (GSEA) to explore the biological functions of ANXA9. The relationship of ANXA9 expression and cancer immune infiltrates was analyzed using the Tumor Immune Estimation Resource (TIMER). In addition, the potential mechanism of ANXA9 in GC was investigated by analyzing its related genes.
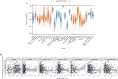
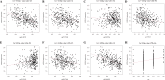
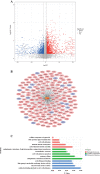
Results: ANXA9 was significantly up-regulated in GC tissues and showed obvious diagnostic value. The expression of ANXA9 was related to the age, gender, grade, TP53 mutation, and histological subtype of GC patients. We also found that ANXA9 expression was associated with immune-related biological function. ANXA9 expression was also correlated with the infiltration level of CD8+ T cells, neutrophils, and dendritic cells in GC. Additionally, copy number variation (VNV) of ANXA9 occurred in GC patients. Function enrichment analyses revealed that ANXA9 plays a role in the GC progression by interacting with its related genes.
Conclusions: Our results provide strong evidence of ANXA9 expression as a prognostic indicator related to immune responses in GC.
Keywords: ANXA9; GEO; Gastric cancer; Immune infiltrates; Prognosis; TCGA.
© 2021 Zhang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Increased IGFBP7 Expression Correlates with Poor Prognosis and Immune Infiltration in Gastric Cancer.J Cancer. 2021 Jan 1;12(5):1343-1355. doi: 10.7150/jca.50370. eCollection 2021. J Cancer. 2021. PMID: 33531979 Free PMC article.
-
BICC1 as a novel prognostic biomarker in gastric cancer correlating with immune infiltrates.Int Immunopharmacol. 2020 Oct;87:106828. doi: 10.1016/j.intimp.2020.106828. Epub 2020 Jul 28. Int Immunopharmacol. 2020. PMID: 32736193
-
Identification and validation of the prognostic value of cyclic GMP-AMP synthase-stimulator of interferon (cGAS-STING) related genes in gastric cancer.Bioengineered. 2021 Dec;12(1):1238-1250. doi: 10.1080/21655979.2021.1911557. Bioengineered. 2021. PMID: 33843442 Free PMC article.
-
Tumor Endothelial Marker TEM7 is a Prognostic Biomarker and Correlating with Immune Infiltrates in Gastric Cancer.Int J Gen Med. 2021 Dec 22;14:10155-10171. doi: 10.2147/IJGM.S347010. eCollection 2021. Int J Gen Med. 2021. PMID: 34992436 Free PMC article.
-
High expression of TMEM200A is associated with a poor prognosis and immune infiltration in gastric cancer.Pathol Oncol Res. 2023 Jan 19;29:1610893. doi: 10.3389/pore.2023.1610893. eCollection 2023. Pathol Oncol Res. 2023. PMID: 36741965 Free PMC article.
Cited by
-
Construction and Validation of a Protein-associated Prognostic Model for Gastrointestinal Cancer.Comb Chem High Throughput Screen. 2023;26(1):191-206. doi: 10.2174/1386207325666220414105743. Comb Chem High Throughput Screen. 2023. PMID: 35430986
-
Harmine loaded Au@MSNs@PEG@Asp6 nano-composites for treatment of spinal metastasis from lung adenocarcinoma by targeting ANXA9 in vivo experiment.Transl Lung Cancer Res. 2023 May 31;12(5):1062-1077. doi: 10.21037/tlcr-23-191. Epub 2023 May 26. Transl Lung Cancer Res. 2023. PMID: 37323183 Free PMC article.
-
Exosome-derived ANXA9 functions as an oncogene in breast cancer.J Pathol Clin Res. 2023 Sep;9(5):378-390. doi: 10.1002/cjp2.334. Epub 2023 Jun 9. J Pathol Clin Res. 2023. PMID: 37294149 Free PMC article.
-
MAM Domain Containing 2 (MAMDC2) Affects Invasion and Metastasis of Human Gastric Cancer.Onco Targets Ther. 2025 May 26;18:679-693. doi: 10.2147/OTT.S516982. eCollection 2025. Onco Targets Ther. 2025. PMID: 40453636 Free PMC article.
-
ANXA9 facilitates S100A4 and promotes breast cancer progression through modulating STAT3 pathway.Cell Death Dis. 2024 Apr 12;15(4):260. doi: 10.1038/s41419-024-06643-4. Cell Death Dis. 2024. PMID: 38609357 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

