Curcumin Oxidation Is Required for Inhibition of Helicobacter pylori Growth, Translocation and Phosphorylation of Cag A
- PMID: 35004346
- PMCID: PMC8740292
- DOI: 10.3389/fcimb.2021.765842
Curcumin Oxidation Is Required for Inhibition of Helicobacter pylori Growth, Translocation and Phosphorylation of Cag A
Abstract
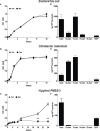
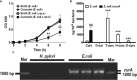
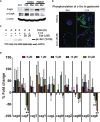
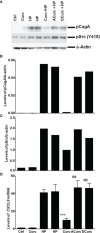
Curcumin is a potential natural remedy for preventing Helicobacter pylori-associated gastric inflammation and cancer. Here, we analyzed the effect of a phospholipid formulation of curcumin on H. pylori growth, translocation and phosphorylation of the virulence factor CagA and host protein kinase Src in vitro and in an in vivo mouse model of H. pylori infection. Growth of H. pylori was inhibited dose-dependently by curcumin in vitro. H. pylori was unable to metabolically reduce curcumin, whereas two enterobacteria, E. coli and Citrobacter rodentium, which efficiently reduced curcumin to the tetra- and hexahydro metabolites, evaded growth inhibition. Oxidative metabolism of curcumin was required for the growth inhibition of H. pylori and the translocation and phosphorylation of CagA and cSrc, since acetal- and diacetal-curcumin that do not undergo oxidative transformation were ineffective. Curcumin attenuated mRNA expression of the H. pylori virulence genes cagE and cagF in a dose-dependent manner and inhibited translocation and phosphorylation of CagA in gastric epithelial cells. H. pylori strains isolated from dietary curcumin-treated mice showed attenuated ability to induce cSrc phosphorylation and the mRNA expression of the gene encoding for IL-8, suggesting long-lasting effects of curcumin on the virulence of H. pylori. Our work provides mechanistic evidence that encourages testing of curcumin as a dietary approach to inhibit the virulence of CagA.
Keywords: CagA; Helicobacter pylori; curcumin; metabolic transformation; reduced curcumin.
Copyright © 2021 Ray, Luis, Mishra, Barry, Asim, Pandey, Chaturvedi, Gupta, Gupta, Mahant, Das, Kumar, Shalimar, Wilson, Schneider and Chaturvedi.
Conflict of interest statement
Author RC was employed by company Nanofludiks Research Pvt. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
The HopQ-CEACAM Interaction Controls CagA Translocation, Phosphorylation, and Phagocytosis of Helicobacter pylori in Neutrophils.mBio. 2020 Feb 4;11(1):e03256-19. doi: 10.1128/mBio.03256-19. mBio. 2020. PMID: 32019805 Free PMC article.
-
Hydrogen Metabolism in Helicobacter pylori Plays a Role in Gastric Carcinogenesis through Facilitating CagA Translocation.mBio. 2016 Aug 16;7(4):e01022-16. doi: 10.1128/mBio.01022-16. mBio. 2016. PMID: 27531909 Free PMC article.
-
Helicobacter pylori virulence factors affecting gastric proton pump expression and acid secretion.Am J Physiol Gastrointest Liver Physiol. 2015 Aug 1;309(3):G193-201. doi: 10.1152/ajpgi.00099.2015. Epub 2015 Jun 4. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 26045613 Free PMC article.
-
[Advances in CagA protein and CagAmediated pathogenesis of Helicobacter pylori-A review].Wei Sheng Wu Xue Bao. 2016 Dec 4;56(12):1821-30. Wei Sheng Wu Xue Bao. 2016. PMID: 29741846 Review. Chinese.
-
CagA-mediated pathogenesis of Helicobacter pylori.Microb Pathog. 2016 Apr;93:44-55. doi: 10.1016/j.micpath.2016.01.005. Epub 2016 Jan 12. Microb Pathog. 2016. PMID: 26796299 Review.
Cited by
-
Advancing Gastrointestinal Health: Curcumin's Efficacy and Nanopreparations.Molecules. 2024 Apr 7;29(7):1659. doi: 10.3390/molecules29071659. Molecules. 2024. PMID: 38611938 Free PMC article. Review.
-
Reactive Oxygen Species and H. pylori Infection: A Comprehensive Review of Their Roles in Gastric Cancer Development.Antioxidants (Basel). 2023 Sep 2;12(9):1712. doi: 10.3390/antiox12091712. Antioxidants (Basel). 2023. PMID: 37760015 Free PMC article. Review.
-
Natural products based on Correa's cascade for the treatment of gastric cancer trilogy: Current status and future perspective.J Pharm Anal. 2025 Feb;15(2):101075. doi: 10.1016/j.jpha.2024.101075. Epub 2024 Aug 19. J Pharm Anal. 2025. PMID: 39957902 Free PMC article. Review.
-
Anticancer applications of phytochemicals in gastric cancer: Effects and molecular mechanism.Front Pharmacol. 2023 Jan 12;13:1078090. doi: 10.3389/fphar.2022.1078090. eCollection 2022. Front Pharmacol. 2023. PMID: 36712679 Free PMC article. Review.
-
Helicobacter pylori infection in humans and phytotherapy, probiotics, and emerging therapeutic interventions: a review.Front Microbiol. 2024 Jan 10;14:1330029. doi: 10.3389/fmicb.2023.1330029. eCollection 2023. Front Microbiol. 2024. PMID: 38268702 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

