Spatiotemporal Expression of SHH/GLI Signaling in Human Fetal Bladder Development
- PMID: 35004540
- PMCID: PMC8727552
- DOI: 10.3389/fped.2021.765255
Spatiotemporal Expression of SHH/GLI Signaling in Human Fetal Bladder Development
Abstract
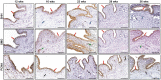
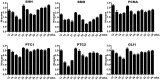
Objectives: Sonic hedgehog (SHH) signaling is important in bladder development. Mice with defective hedgehog signaling develop bladder anomalies. Clinically, urinary tract malformations are reported in human fetuses and infants with mutations of SHH and related signaling pathway genes. Information on the expression of SHH and associated signaling genes in normal human bladder development is fragmentary. This study determined the temporal and spatial expression patterns of SHH signaling pathway components in human fetal bladders by immunohistochemistry (IHC). Material and Methods: Twenty-four bladder specimens from 16 male and 8 female human fetuses aged 12- to 36-week (wk) were obtained from the First Affiliated Hospital of Xi'an Jiaotong University. The tissue slides were processed for IHC staining with SHH, Patched1 (PTC-1), Patched2 (PTC-2), Smoothened (SMO), GLI1 and proliferating cell nuclear antigen (PCNA). The expression levels of each gene were analyzed by semi-quantitative histological scoring system. Results: High intensity of SHH and SMO expression was detected in developing bladder urothelial cells, with no staining in lamina propria (LP), but with minimal expression of SMO in differentiating smooth muscle (SM) layers. The spatial distribution pattern of PTC1 and GLI1 was more complex with minimal expression in the LP layer, moderate expression in the SM layer, and high expression in the urothelium. PTC2 expression was mainly localized in the urothelium and LP, but no expression in the SM layer. All of the SHH signaling components were detected in fetal bladder tissues throughout the development, with expression peaks at 12- and 23-wk, coinciding with high cell proliferation as indicated by PCNA staining in the cell nuclei of urothelium and SM. Conclusions: The autocrine SHH signaling in the developing urothelium, and paracrine SHH signaling in the developing smooth muscle layer, mediated by SMO, PTC-1 and GLI1 were demonstrated during human bladder development. Expression of SHH signaling components peaked at 12-and 23-wk. The first expression peak at 12-wk may relate to urothelium growth, SM induction, and dilation of the bladder cavity. The second expression peaked at 23-wk may relate to urothelium and SM layer differentiation.
Keywords: GLI1; bladder development; patched; smoothened (SMO); sonic hedgehog signaling.
Copyright © 2021 Zhang, Xu, He, Wang and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Urothelial sonic hedgehog signaling plays an important role in bladder smooth muscle formation.Differentiation. 2007 Dec;75(10):968-77. doi: 10.1111/j.1432-0436.2007.00187.x. Epub 2007 May 9. Differentiation. 2007. PMID: 17490411
-
Sonic and desert hedgehog signaling in human fetal prostate development.Prostate. 2007 May 1;67(6):674-84. doi: 10.1002/pros.20563. Prostate. 2007. PMID: 17342747
-
Immunohistochemical analysis of Sonic hedgehog signalling in normal human urinary tract development.J Anat. 2007 Nov;211(5):620-9. doi: 10.1111/j.1469-7580.2007.00808.x. Epub 2007 Sep 11. J Anat. 2007. PMID: 17850284 Free PMC article.
-
Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors.Cancers (Basel). 2016 Feb 15;8(2):22. doi: 10.3390/cancers8020022. Cancers (Basel). 2016. PMID: 26891329 Free PMC article. Review.
-
Small-molecule modulators of the Sonic Hedgehog signaling pathway.Mol Biosyst. 2010 Jan;6(1):44-54. doi: 10.1039/b910196a. Epub 2009 Aug 27. Mol Biosyst. 2010. PMID: 20024066 Review.
Cited by
-
Interstitial Cystitis: a phenotype and rare variant exome sequencing study: Interstitial Cystitis: a phenotype and exome sequencing study.medRxiv [Preprint]. 2025 Feb 18:2025.02.16.25322147. doi: 10.1101/2025.02.16.25322147. medRxiv. 2025. PMID: 40034785 Free PMC article. Preprint.
-
Novel urinary tract obstruction marker discovery by multi-marker profiling of urinary extracellular vesicles derived from a rat UTO model.Am J Clin Exp Urol. 2023 Apr 15;11(2):136-145. eCollection 2023. Am J Clin Exp Urol. 2023. PMID: 37168944 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

