Technological Approaches in the Analysis of Extracellular Vesicle Nucleotide Sequences
- PMID: 35004647
- PMCID: PMC8733665
- DOI: 10.3389/fbioe.2021.787551
Technological Approaches in the Analysis of Extracellular Vesicle Nucleotide Sequences
Abstract
Together with metabolites, proteins, and lipid components, the EV cargo consists of DNA and RNA nucleotide sequence species, which are part of the intracellular communication network regulating specific cellular processes and provoking distinct target cell responses. The extracellular vesicle (EV) nucleotide sequence cargo molecules are often investigated in association with a particular pathology and may provide an insight into the physiological and pathological processes in hard-to-access organs and tissues. The diversity and biological function of EV nucleotide sequences are distinct regarding EV subgroups and differ in tissue- and cell-released EVs. EV DNA is present mainly in apoptotic bodies, while there are different species of EV RNAs in all subgroups of EVs. A limited sample volume of unique human liquid biopsy provides a small amount of EVs with limited isolated DNA and RNA, which can be a challenging factor for EV nucleotide sequence analysis, while the additional difficulty is technical variability of molecular nucleotide detection. Every EV study is challenged with its first step of the EV isolation procedure, which determines the EV's purity, yield, and diameter range and has an impact on the EV's downstream analysis with a significant impact on the final result. The gold standard EV isolation procedure with ultracentrifugation provides a low output and not highly pure isolated EVs, while modern techniques increase EV's yield and purity. Different EV DNA and RNA detection techniques include the PCR procedure for nucleotide sequence replication of the molecules of interest, which can undergo a small-input EV DNA or RNA material. The nucleotide sequence detection approaches with their advantages and disadvantages should be considered to appropriately address the study problem and to extract specific EV nucleotide sequence information with the detection using qPCR or next-generation sequencing. Advanced next-generation sequencing techniques allow the detection of total EV genomic or transcriptomic data even at the single-molecule resolution and thus, offering a sensitive and accurate EV DNA or RNA biomarker detection. Additionally, with the processes where the EV genomic or transcriptomic data profiles are compared to identify characteristic EV differences in specific conditions, novel biomarkers could be discovered. Therefore, a suitable differential expression analysis is crucial to define the EV DNA or RNA differences between conditions under investigation. Further bioinformatics analysis can predict molecular cell targets and identify targeted and affected cellular pathways. The prediction target tools with functional studies are essential to help specify the role of the investigated EV-targeted nucleotide sequences in health and disease and support further development of EV-related therapeutics. This review will discuss the biological diversity of human liquid biopsy-obtained EV nucleotide sequences DNA and RNA species reported as potential biomarkers in health and disease and methodological principles of their detection, from human liquid biopsy EV isolation, EV nucleotide sequence extraction, techniques for their detection, and their cell target prediction.
Keywords: DNA; RNA; biomarkers; extracellular vesicles (EVs); nucleotide sequences detection; therapeutics.
Copyright © 2021 Tesovnik, Jenko Bizjan, Šket, Debeljak, Battelino and Kovač.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

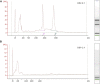
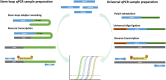

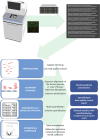


References
Publication types
LinkOut - more resources
Full Text Sources

