Methanol Dehydrogenases as a Key Biocatalysts for Synthetic Methylotrophy
- PMID: 35004648
- PMCID: PMC8741260
- DOI: 10.3389/fbioe.2021.787791
Methanol Dehydrogenases as a Key Biocatalysts for Synthetic Methylotrophy
Abstract
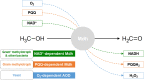
One-carbon (C1) chemicals are potential building blocks for cheap and sustainable re-sources such as methane, methanol, formaldehyde, formate, carbon monoxide, and more. These resources have the potential to be made into raw materials for various products used in our daily life or precursors for pharmaceuticals through biological and chemical processes. Among the soluble C1 substrates, methanol is regarded as a biorenewable platform feedstock because nearly all bioresources can be converted into methanol through syngas. Synthetic methylotrophy can be exploited to produce fuels and chemicals using methanol as a feedstock that integrates natural or artificial methanol assimilation pathways in platform microorganisms. In the methanol utilization in methylotrophy, methanol dehydrogenase (Mdh) is a primary enzyme that converts methanol to formaldehyde. The discovery of new Mdhs and engineering of present Mdhs have been attempted to develop synthetic methylotrophic bacteria. In this review, we describe Mdhs, including in terms of their enzyme properties and engineering for desired activity. In addition, we specifically focus on the application of various Mdhs for synthetic methylotrophy.
Keywords: C1 gas; assimilation; formaldehyde; methanol dehydrogenase; synthetic methylotrophy.
Copyright © 2021 Le, Lee, Han and Yeom.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Anthony C. (1982). The Biochemistry of Methylotrophs. London, England: Academic Press.
Publication types
LinkOut - more resources
Full Text Sources