COVID-19 Pandemic and Trends in Clinical Trials: A Multi-Region and Global Perspective
- PMID: 35004791
- PMCID: PMC8739772
- DOI: 10.3389/fmed.2021.812370
COVID-19 Pandemic and Trends in Clinical Trials: A Multi-Region and Global Perspective
Abstract
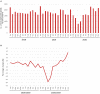
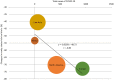
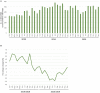
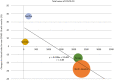
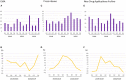
To evaluate the effect of the COVID-19 pandemic on clinical development, the number of newly started clinical trials in each geographical region between January 2018 and December 2020 were calculated based on data from the ClinicalTrials.gov database. Data regarding new drug applications were obtained from European Medicines Agency monthly reports, pharmaceutical company press releases, and the archives of the Drugs.com database. The mean percentage change in newly started clinical trials for diseases other than COVID-19 between each month in 2019 and the corresponding month in 2020 was -7.5%, with the maximum of -57.3% observed between April 2019 and April 2020. Similarly, the mean percentage change of reported results for each month in 2019 and 2020 was -5.1%, with the maximum of -27.4% observed in July 2020. The activity of clinical trials was decreased as the number of COVID-19 patients was increased, and a statistically negative correlation was observed between the prevalence of COVID-19 and the percentage decrease in the number of clinical trials stared or reported results. As for new drug submissions, decreases were observed in the latter half of 2020 compared with the same period during the previous year, for each indicator. A considerable decline in non-COVID-19 activity for all indicators regarding clinical developments was suggested during the first wave of the COVID-19 pandemic. It is important to recognize the situation and continue to make efforts to conduct clinical trials for both COVID-19 and no-COVID-19 for new medical developments in the future.
Keywords: COVID-19 pandemic; clinical development; clinical trial; new drug application; non-COVID-19 activity.
Copyright © 2021 Nishiwaki and Ando.
Conflict of interest statement
YA reports grants and personal fees from Chugai Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Nippon Kayaku Co., Ltd., Yakult Honsha Co., Ltd., Eli Lilly Japan K.K., Mochida Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., and Taiho Pharmaceutical Co., Ltd., personal fees from Novartis Pharma K.K., Merck Serono Co., Ltd., Bayer Holding Ltd., Bristol-Myers Squibb, Otsuka Holdings Co., Ltd., and Sawai Pharmaceutical Co., Ltd., grants and personal fees from Daiichi Sankyo Company, Ltd., grants from Eisai Co., Ltd., Hisamitsu Pharmaceutical Co., Inc., and Takeda Pharmaceutical Co., Ltd., personal fees from Tsumura & Co., Shionogi & Co., Ltd., Janssen Pharmaceutical K.K., and Pharma International Inc., outside the submitted work. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- US . Food and Drug Administration. FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Public Health Emergency: Guidance for Industry, Investigators, and Institutional Review Boards. Available online at: https://wwwfdagov/regulatory-information/search-fda-guidance-documents/f... (accessed January 22, 2021).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

