Boosting Vaccine-Elicited Respiratory Mucosal and Systemic COVID-19 Immunity in Mice With the Oral Lactobacillus plantarum
- PMID: 35004816
- PMCID: PMC8733898
- DOI: 10.3389/fnut.2021.789242
Boosting Vaccine-Elicited Respiratory Mucosal and Systemic COVID-19 Immunity in Mice With the Oral Lactobacillus plantarum
Abstract
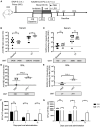
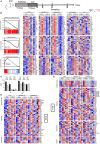
Boosting and prolonging SARS-CoV-2 vaccine-elicited immunity is paramount for containing the COVID-19 pandemic, which wanes substantially within months after vaccination. Here we demonstrate that the unique strain of probiotic Lactobacillus plantarum GUANKE (LPG) could promote SARS-CoV-2-specific immune responses in both effective and memory phases through enhancing interferon signaling and suppressing apoptotic and inflammatory pathways. Interestingly, oral LPG administration promoted SARS-CoV-2 neutralization antibodies even 6 months after immunization. Furthermore, when LPG was given immediately after SARS-CoV-2 vaccine inoculation, specific neutralization antibodies could be boosted >8-fold in bronchoalveolar lavage (BAL) and >2-fold in sera, T-cell responses were persistent and stable for a prolonged period both in BAL and the spleen. Transcriptional analyses showed that oral application of LPG mobilized immune responses in the mucosal and systemic compartments; in particular, gut-spleen and gut-lung immune axes were observed. These results suggest that LPG could be applied in combination with SARS-CoV-2 vaccines to boost and prolong both the effective and memory immune responses in mucosal and systemic compartments, thereby improving the efficacy of SARS-CoV-2 vaccination.
Keywords: COVID-19; Lactobacillus plantarum; accquired immunity; adjuvant; gut-lung axis; memory immunity; probiotics; vaccine.
Copyright © 2021 Xu, Ren, Cao, Li, Yang, Luo, Zhu, Wang, Ding, Liang, Jin, Yuan, Li and Xu.
Conflict of interest statement
JGX, JQX, ZHR, and KLC filed patent describing the invention and use of the probiotic described in the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Comment in
-
Commentary: Boosting Vaccine-Elicited Respiratory Mucosal and Systemic COVID-19 Immunity in Mice With the Oral Lactobacillus plantarum.Front Nutr. 2022 Feb 18;9:846379. doi: 10.3389/fnut.2022.846379. eCollection 2022. Front Nutr. 2022. PMID: 35252314 Free PMC article. No abstract available.
References
-
- The COVID-19 vaccine race–weekly update . Available online at: http://www.gavi.org/vaccineswork/covid-19-vaccine-race
-
- Dosoky NS, Chen Z, Guo Y, McMillan C, Flynn CR, Davies SS. Two-week administration of engineered Escherichia coli establishes persistent resistance to diet-induced obesity even without antibiotic pre-treatment. Appl Microbiol Biotechnol. (2019) 103:6711–23. 10.1007/s00253-019-09958-x - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

