A Sulfuryl Group Transfer Strategy to Selectively Prepare Sulfated Steroids and Isotopically Labelled Derivatives
- PMID: 35004848
- PMCID: PMC8740147
- DOI: 10.3389/fmolb.2021.776900
A Sulfuryl Group Transfer Strategy to Selectively Prepare Sulfated Steroids and Isotopically Labelled Derivatives
Erratum in
-
Corrigendum: A sulfuryl group transfer strategy to selectively prepare sulfated steroids and isotopically labelled derivatives.Front Mol Biosci. 2024 Dec 19;11:1504226. doi: 10.3389/fmolb.2024.1504226. eCollection 2024. Front Mol Biosci. 2024. PMID: 39749217 Free PMC article.
Abstract
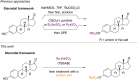
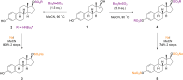
The treatment of common steroids: estrone, estradiol, cortisol, and pregnenolone with tributylsulfoammonium betaine (TBSAB) provides a convenient chemoselective conversion of the steroids alcohol/phenol moiety to the corresponding steroidal organosulfate. An important feature of the disclosed methodology is the millimolar scale of the reaction, and the isolation of the corresponding steroid sulfates as their biologically relevant sodium salts without the need for ion-exchange chromatography. The scope of the method was further explored in the estradiol and pregnanediol steroid systems with the bis-sulfated derivatives. Ultimately, a method to install an isotopic label, deuterium (2H) combined with estrone sulfation is a valuable tool for its mass-spectrometric quantification in biological studies.
Keywords: TBSAB; isotopic labelling; selectivity; sulfation; sulfuryl transfer.
Copyright © 2021 Alshehri, Gill and Jones.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- CCDC 2120263 Contains the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge from the Cambridge Crystallographic Data Centre. Available at: www.ccdc.cam.ac.uk/data_request/cif .
-
- Gabriel L., Günther W., Pielenz F., Heinze T. (2020). Determination of the Binding Situation of Pyridine in Xylan Sulfates by Means of Detailed NMR Studies. Macromol. Chem. Phys. 221, 1900327. 10.1002/macp.201900327 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

