Real-World Anticoagulatory Treatment After Transcatheter Aortic Valve Replacement: A Retrospective, Observational Study on 4,800 Patients
- PMID: 35004894
- PMCID: PMC8733398
- DOI: 10.3389/fcvm.2021.780762
Real-World Anticoagulatory Treatment After Transcatheter Aortic Valve Replacement: A Retrospective, Observational Study on 4,800 Patients
Abstract
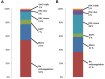
Background: Transcatheter aortic valve replacement (TAVR) has developed to the therapy of choice for patients with symptomatic severe aortic stenosis who are unsuitable for surgical aortic valve replacement and elderly patients with intermediate or high operative risk. However, the optimal anticoagulant therapy post-TAVR still remains a matter of debate. Aims: This study sought to investigate current anticoagulant treatment patterns and clinical outcome in patients undergoing TAVR. Methods: In a retrospective study based on anonymized health claims data of approximately seven million Germans with statutory health insurance (InGef database), anticoagulant treatment regimens were assessed using any drug prescription post discharge within the first 90 days after TAVR procedure. Clinical events between 30 days and 6 months were examined by treatment regime. Results: The study population comprised 4,812 patients with TAVR between 2014 and 2018: 29.4% received antiplatelet monotherapy, 17.8% dual antiplatelet therapy, 17.4% oral anticoagulation (OAC) plus antiplatelet therapy, 12.9% OAC monotherapy, 2.2% triple therapy and 19.2% did not receive any anticoagulatory drugs. Sixty-four percentage of patients with OAC received direct oral anticoagulants (DOAC). Hence, 68% of all patients were treated non-adherent to current guidelines. Forty percentage of patients with OAC prior to TAVR did not have any OAC after TAVR. The adjusted risk of all-cause mortality was significantly increased in patients with OAC (HR 1.40, 95% CI 1.03-1.90, p = 0.03) and no anticoagulatory treatment (HR 3.95, 95% CI 2.95-5.27, p < 0.0001) when compared to antiplatelet monotherapy. Conclusions: This large real-world data analysis demonstrates substantial deviations from guideline recommendations and treatment after TAVR. Considering relevant differences in clinical outcome across treatment groups, major effort is warranted to examine underlying causes and improve guideline adherence.
Keywords: DOAC; TAVR; anticoagulation; antithrombotic therapy; real-world.
Copyright © 2021 Hohmann, Ludwig, Walker, Wienemann, Baldus and Pfister.
Conflict of interest statement
ML and JW were employed by company InGef-Institute for Applied Health Research Berlin GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Real-world anticoagulatory treatment after percutaneous mitral valve repair using MitraClip: a retrospective, observational study on 1300 patients.Clin Res Cardiol. 2022 Aug;111(8):889-899. doi: 10.1007/s00392-022-01988-2. Epub 2022 Feb 26. Clin Res Cardiol. 2022. PMID: 35220447 Free PMC article.
-
Challenges in Aortic Stenosis: Review of Antiplatelet/Anticoagulant Therapy Management with Transcatheter Aortic Valve Replacement (TAVR): TAVR with Recent PCI, TAVR in the Patient with Atrial Fibrillation, and TAVR Thrombosis Management.Curr Cardiol Rep. 2018 Oct 11;20(12):130. doi: 10.1007/s11886-018-1073-9. Curr Cardiol Rep. 2018. PMID: 30311013 Review.
-
Antithrombotic Therapy After Transcatheter Aortic Valve Replacement.JACC Cardiovasc Interv. 2021 Aug 9;14(15):1688-1703. doi: 10.1016/j.jcin.2021.06.020. JACC Cardiovasc Interv. 2021. PMID: 34353601 Review.
-
Antithrombotic Therapy after Transcatheter Aortic Valve Replacement.Heart Views. 2022 Jan-Mar;23(1):10-15. doi: 10.4103/heartviews.heartviews_36_22. Epub 2022 May 16. Heart Views. 2022. PMID: 35757447 Free PMC article. Review.
-
Comparison of dual antiplatelet therapy versus oral anticoagulation following transcatheter aortic valve replacement: A retrospective single-center registry analysis.Cardiol J. 2017;24(6):649-659. doi: 10.5603/CJ.a2017.0050. Epub 2017 May 12. Cardiol J. 2017. PMID: 28497845
Cited by
-
Efficacy and outcomes of antiplatelet therapy versus oral anticoagulants in patients undergoing transcatheter aortic valve replacement: a systematic review and meta-analysis.Ann Med Surg (Lond). 2024 Mar 15;86(5):2911-2925. doi: 10.1097/MS9.0000000000001908. eCollection 2024 May. Ann Med Surg (Lond). 2024. PMID: 38694361 Free PMC article. Review.
-
Unusual Complications after Transcatheter Aortic Valve Replacement: Gastrointestinal Bleeding in an Elderly Patient with Previous Coronary Artery Bypass Graft: A Case Report.Adv Biomed Res. 2025 Jul 21;14:60. doi: 10.4103/abr.abr_416_24. eCollection 2025. Adv Biomed Res. 2025. PMID: 40862182 Free PMC article.
References
LinkOut - more resources
Full Text Sources

