Comparison of Gut Microbiota and Metabolic Status of Sows With Different Litter Sizes During Pregnancy
- PMID: 35004929
- PMCID: PMC8733392
- DOI: 10.3389/fvets.2021.793174
Comparison of Gut Microbiota and Metabolic Status of Sows With Different Litter Sizes During Pregnancy
Abstract
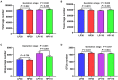
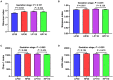
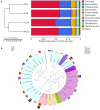
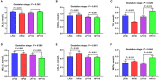
The experiment was conducted to compare the differences of gut microbiota and metabolic status of sows with different litter sizes on days 30 and 110 of gestation, and uncover the relationship between the composition of maternal gut microbiota during gestation and sow reproductive performance. Twenty-six Large White × Landrace crossbred multiparous sows (2nd parity) with similar back fat thickness and body weight were assigned to two groups [high-reproductive performance group (HP group) and low-reproductive performance group (LP group)] according to their litter sizes and fed a common gestation diet. Results showed that compared with LP sows, HP sows had significantly lower plasma levels of triglyceride (TG) on gestation d 30 (P < 0.05), but had significantly higher plasma levels of TG, non-esterified fatty acid, tumor necrosis factor-α, and immunoglobulin M on gestation d 110 (P < 0.05). Consistently, HP sows revealed increased alpha diversity and butyrate-producing genera, as well as fecal butyrate concentration, on gestation d 30; HP sows showed significantly different microbiota community structure with LP sows (P < 0.05) and had markedly higher abundance of Firmicutes (genera Christensenellaceae_R-7_group and Terrisporobacter) which were positively related with litter size on gestation d 110 than LP sows (P < 0.05). In addition, plasma biochemical parameters, plasma cytokines, and fecal microbiota shifted dramatically from gestation d 30 to d 110. Therefore, our findings demonstrated that microbial abundances and community structures differed significantly between sows with different litter sizes and gestation stages, which was associated with changes in plasma biochemical parameters, inflammatory factors, and immunoglobulin. Moreover, these findings revealed that there was a significant correlation between litter size and gut microbiota of sows, and provided a microbial perspective to improve sow reproductive performance in pig production.
Keywords: gestation stage; gut microbiota; litter size; metabolic status; reproductive performance; sow.
Copyright © 2021 Chen, Li, Yang, Jiang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

