STARD1: a new rising StAR in cholesterol-mediated hepatocarcinogenesis
- PMID: 35004970
- PMCID: PMC8683904
- DOI: 10.21037/hbsn-21-374
STARD1: a new rising StAR in cholesterol-mediated hepatocarcinogenesis
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/hbsn-21-374). The authors have no conflicts of interest to declare.
Figures
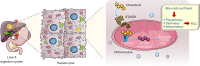
Comment on
-
STARD1 promotes NASH-driven HCC by sustaining the generation of bile acids through the alternative mitochondrial pathway.J Hepatol. 2021 Jun;74(6):1429-1441. doi: 10.1016/j.jhep.2021.01.028. Epub 2021 Jan 27. J Hepatol. 2021. PMID: 33515644 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
