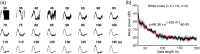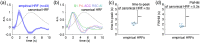Computing hemodynamic response functions from concurrent spectral fiber-photometry and fMRI data
- PMID: 35005057
- PMCID: PMC8734587
- DOI: 10.1117/1.NPh.9.3.032205
Computing hemodynamic response functions from concurrent spectral fiber-photometry and fMRI data
Abstract
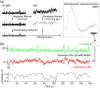
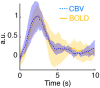
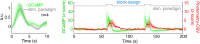
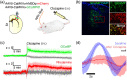
Significance: Although emerging evidence suggests that the hemodynamic response function (HRF) can vary by brain region and species, a single, canonical, human-based HRF is widely used in animal studies. Therefore, the development of flexible, accessible, brain-region specific HRF calculation approaches is paramount as hemodynamic animal studies become increasingly popular. Aim: To establish an fMRI-compatible, spectral, fiber-photometry platform for HRF calculation and validation in any rat brain region. Approach: We used our platform to simultaneously measure (a) neuronal activity via genetically encoded calcium indicators (GCaMP6f), (b) local cerebral blood volume (CBV) from intravenous Rhodamine B dye, and (c) whole brain CBV via fMRI with the Feraheme contrast agent. Empirical HRFs were calculated with GCaMP6f and Rhodamine B recordings from rat brain regions during resting-state and task-based paradigms. Results: We calculated empirical HRFs for the rat primary somatosensory, anterior cingulate, prelimbic, retrosplenial, and anterior insular cortical areas. Each HRF was faster and narrower than the canonical HRF and no significant difference was observed between these cortical regions. When used in general linear model analyses of corresponding fMRI data, the empirical HRFs showed better detection performance than the canonical HRF. Conclusions: Our findings demonstrate the viability and utility of fiber-photometry-based HRF calculations. This platform is readily scalable to multiple simultaneous recording sites, and adaptable to study transfer functions between stimulation events, neuronal activity, neurotransmitter release, and hemodynamic responses.
Keywords: Fiber-photometry; Hemodynamic response function; MRI compatible; fMRI; multi-modal; rat.
© 2022 The Authors.
Figures