A Phenotypic Screen for the Liver Stages of Plasmodium vivax
- PMID: 35005096
- PMCID: PMC8678555
- DOI: 10.21769/BioProtoc.4253
A Phenotypic Screen for the Liver Stages of Plasmodium vivax
Abstract
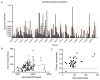
Control of malaria caused by Plasmodium vivax can be improved by the discovery and development of novel drugs against the parasite's liver stage, which includes relapse-causing hypnozoites. Several recent reports describe breakthroughs in the culture of the P. vivax liver stage in 384-well microtiter plates, with the goal of enabling a hypnozoite-focused drug screen. Herein we describe assay details, protocol developments, and different assay formats to interrogate the chemical sensitivity of the P. vivax liver stage in one such medium-throughput platform. The general assay protocol includes seeding of primary human hepatocytes which are infected with P. vivax sporozoites generated from the feeding of Anopheles dirus mosquitoes on patient isolate bloodmeals. This protocol is unique in that, after source drug plates are supplied, all culture-work steps have been optimized to preclude the need for automated liquid handling, thereby allowing the assay to be performed within resource-limited laboratories in malaria-endemic countries. Throughput is enhanced as complex culture methods, such as extracellular matrix overlays, multiple cell types in co-culture, or hepatic spheroids, are excluded as the workflow consists entirely of routine culture methods for adherent cells. Furthermore, installation of a high-content imager at the study site enables assay data to be read and transmitted with minimal logistical delays. Herein we detail distinct assay improvements which increase data quality, provide a means to limit the confounding effect of hepatic metabolism on assay data, and detect activity of compounds with a slow-clearance phenotype. Graphical abstract: Overview of P. vivax liver stage screening assay performed at the Institute Pasteur of Cambodia.
Keywords: 8-aminoquinolines; Antirelapse; Hypnozoites; Liver stage assay; Phenotypic screening; Plasmodium vivax; Primaquine; Tafenoquine.
Copyright © 2021 The Authors; exclusive licensee Bio-protocol LLC.
Conflict of interest statement
Competing interestsThe authors have no competing interests to declare.
Figures










References
-
- Bousema T., Dinglasan R. R., Morlais I., Gouagna L. C., van Warmerdam T., Awono-Ambene P. H., Bonnet S., Diallo M., Coulibaly M., Tchuinkam T., et al. .(2012). Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers . PLoS One 7(8): e42821. - PMC - PubMed
-
- Chu C. S., Bancone G., Moore K. A., Win H. H., Thitipanawan N., Po C., Chowwiwat N., Raksapraidee R., Wilairisak P., Phyo A. P., et al. .(2017). Haemolysis in G6PD Heterozygous Females Treated with Primaquine for Plasmodium vivax Malaria: A Nested Cohort in a Trial of Radical Curative Regimens . PLoS Med 14(2): e1002224. - PMC - PubMed
-
- Chua A. C. Y., Ananthanarayanan A., Ong J. J. Y., Wong J. Y., Yip A., Singh N. H., Qu Y., Dembele L., McMillian M., Ubalee R., et al. .(2019). Hepatic spheroids used as an in vitro model to study malaria relapse . Biomaterials 216: 119221. - PubMed
-
- Dembele L., Franetich J. F., Lorthiois A., Gego A., Zeeman A. M., Kocken C. H., Le Grand R., Dereuddre-Bosquet N., van Gemert G. J., Sauerwein R., et al. .(2014). Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat Med 20(3): 307-312. - PubMed
LinkOut - more resources
Full Text Sources

