Dermatologic immune-related adverse events: The toxicity spectrum and recommendations for management
- PMID: 35005180
- PMCID: PMC8721136
- DOI: 10.1016/j.ijwd.2021.10.005
Dermatologic immune-related adverse events: The toxicity spectrum and recommendations for management
Abstract
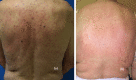
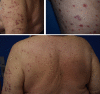
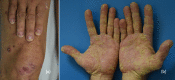
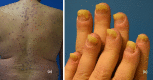
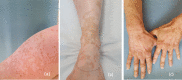
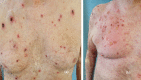
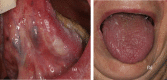
Immune checkpoint inhibitors are a new class of oncologic drugs that act via the inhibition of checkpoints, thereby unlocking the immune system to attack cancer cells. Their emergence has radically changed the concept of therapy in oncologic patients. However, despite their overall favorable profile, their use has been associated with specific toxicities that may potentially affect treatment. The so-called immune-related adverse events (irAEs) mostly correspond to dysimmune reactions that can affect nearly every organ system, in theory, notably with the development of colitis, hepatitis, pneumonitis, or thyroiditis. Dermatologic irAEs are also among the most common, reaching a rate of approximately 40%. They are characterized by a wide phenotypic range, including mainly eczematous or lichenoid rashes, psoriasis, or autoimmune bullous disorders. Pruritus may accompany the aforementioned rashes or develop as an isolated symptom without the presence of skin changes. Depigmentation and hair/nail changes can be also observed in association with immune checkpoint inhibitor treatment. In the current article, we present an overview of the clinical spectrum of irAEs and provide tips for early recognition and management of dermatologic irAEs. We highlight the role that dermatologists can play in relieving patients and allowing for oncologic treatment to be maintained and administered more safely.
Keywords: Immune checkpoint inhibitors; adverse events; pruritus; rash; skin toxicity.
© 2021 Published by Elsevier Inc. on behalf of Women's Dermatologic Society.
Figures

References
-
- Antoury L, Maloney NJ, Bach DQ, Goh C, Cheng K. Alopecia areata as an immune-related adverse event of immune checkpoint inhibitors: A review. Dermatol Ther. 2020;33(6):e14171. - PubMed
-
- Apalla Z, Kemanetzi C, Papageorgiou C, Bobos M, Manoli M, Fotiadou C, et al. Challenges in sarcoidosis and sarcoid-like reactions associated to immune checkpoint inhibitors: A narrative review apropos of a case. Dermatol Ther. 2021;34(1):e14618. - PubMed
-
- Apalla Z, Lallas A, Delli F, Lazaridou E, Papalampou S, Apostolidou S, et al. Management of immune checkpoint inhibitor-induced bullous pemphigoid. J Am Acad Dermatol. 2021;84(2):540–543. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

