Cerebrovascular stiffness and flow dynamics in the presence of amyloid and tau biomarkers
- PMID: 35005194
- PMCID: PMC8719432
- DOI: 10.1002/dad2.12253
Cerebrovascular stiffness and flow dynamics in the presence of amyloid and tau biomarkers
Abstract
Introduction: This work investigated the relationship between cerebrovascular disease (CVD) markers and Alzheimer's disease (AD) biomarkers of amyloid beta deposition, and neurofibrillary tau tangles in subjects spanning the AD clinical spectrum.
Methods: A total of 136 subjects participated in this study. Four groups were established based on AD biomarker positivity from positron emission tomography (amyloid [A] and tau [T]) and clinical diagnosis (cognitively normal [CN] and impaired [IM]). CVD markers were derived from structural and quantitative magnetic resonance imaging data.
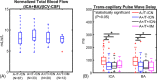
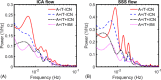
Results: Transcapillary pulse wave delay was significantly longer in controls compared to AT biomarker-confirmed groups (A+/T-/CN P < .001, A+/T+/CN P < .001, A+/T+/IM P = .003). Intracranial low-frequency oscillations were diminished in AT biomarker-confirmed groups both CN and impaired (A+/T-/CN P = .039, A+/T+/CN P = .007, A+/T+/IM P = .011). A significantly higher presence of microhemorrhages was measured in A+/T+/CN compared to controls (P = .006).
Discussion: Cerebrovascular markers indicate increased vessel stiffness and reduced vasomotion in AT biomarker-positive subjects during preclinical AD.
Keywords: Alzheimer's disease; amyloid imaging; low‐frequency oscillations; vascular imaging; vessel stiffness.
© 2021 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
K.A. Cody, T. Reher, and R.V. Cadman have nothing to disclose. L.A. Rivera‐Rivera's effort on this work was supported by the Alzheimer's Association AARFD‐20‐678095 paid to the institution. L. Eisenmenger's effort on this work was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373 and KL2TR002374, as well as the Wisconsin Alzheimer's Disease Research Center grant P30‐AG062715 paid to the institution. T. Betthauser has also received grant support outside the submitted work from Alzheimer's Association AARF‐19‐614533 paid to the institution. H.A. Rowley has received consulting fees from GE HealthCare, iSchemaView and for accredited lectures and podcasts for Northwest Imaging Forums and the Data Safety Monitoring Board of HL Gore. H.A. Rowley has also received grant support outside the submitted work from the Radiological Society of North America, Fellow Grant paid to the institution. C.M. Carlsson has received payments for her participation in an NIH study at UCLA (D‐CARE) and study drugs from Amarin Corporation (Vascepa and placebo) for VA Merit Study. C.M. Carlsson has also received grant support outside the submitted work from NIH/Lilly, NIH/Esai, VA Merit, and NIH to the institution. N.A. Chin has received payments for being a member of the Med‐Sci committee for the WI Alzheimer's Association and member of the Med‐Sci committee for the Alzheimer's Foundation of America. S.C. Johnson serves on an advisory board for Roche Diagnostics for which he receives an honorarium and is principal investigator of an equipment grant from Roche. He receives research funding from Cerveau Technologies. K.M. Johnson has received royalties for patents licensed by the institution (UW‐Madison) unrelated to this project. K.M. Johnson has also received grant support outside the submitted work from NIH and GE Healthcare as research support to the institution.
Figures


References
-
- Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. The Lancet Neurol. 2013;12(4):357‐367. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
