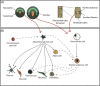
Cell sources proposed for nucleus pulposus regeneration
- PMID: 35005441
- PMCID: PMC8717099
- DOI: 10.1002/jsp2.1175
Cell sources proposed for nucleus pulposus regeneration
Abstract
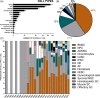
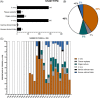
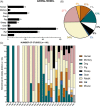
Lower back pain (LBP) occurs in 80% of adults in their lifetime; resulting in LBP being one of the biggest causes of disability worldwide. Chronic LBP has been linked to the degeneration of the intervertebral disc (IVD). The current treatments for chronic back pain only provide alleviation of symptoms through pain relief, tissue removal, or spinal fusion; none of which target regenerating the degenerate IVD. As nucleus pulposus (NP) degeneration is thought to represent a key initiation site of IVD degeneration, cell therapy that specifically targets the restoration of the NP has been reviewed here. A literature search to quantitatively assess all cell types used in NP regeneration was undertaken. With key cell sources: NP cells; annulus fibrosus cells; notochordal cells; chondrocytes; bone marrow mesenchymal stromal cells; adipose-derived stromal cells; and induced pluripotent stem cells extensively analyzed for their regenerative potential of the NP. This review highlights: accessibility; expansion capability in vitro; cell survival in an IVD environment; regenerative potential; and safety for these key potential cell sources. In conclusion, while several potential cell sources have been proposed, iPSC may provide the most promising regenerative potential.
Keywords: biologic therapies; regenerative medicine; stem cell; tissue engineering.
© 2021 The Authors. JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

