GS-CA1 and lenacapavir stabilize the HIV-1 core and modulate the core interaction with cellular factors
- PMID: 35005542
- PMCID: PMC8718827
- DOI: 10.1016/j.isci.2021.103593
GS-CA1 and lenacapavir stabilize the HIV-1 core and modulate the core interaction with cellular factors
Abstract
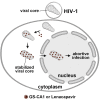
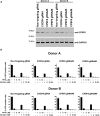
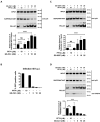
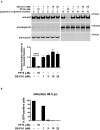
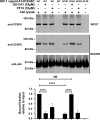
The HIV-1 capsid is the target for the antiviral drugs GS-CA1 and Lenacapavir (GS-6207). We investigated the mechanism by which GS-CA1 and GS-6207 inhibit HIV-1 infection. HIV-1 inhibition by GS-CA1 did not require CPSF6 in CD4+ T cells. Contrary to PF74 that accelerates uncoating of HIV-1, GS-CA1 and GS-6207 stabilized the core. GS-CA1, unlike PF74, allowed the core to enter the nucleus, which agrees with the fact that GS-CA1 inhibits infection after reverse transcription. Unlike PF74, GS-CA1 did not disaggregate preformed CPSF6 complexes in nuclear speckles, suggesting that PF74 and GS-CA1 have different mechanisms of action. GS-CA1 stabilized the HIV-1 core, possibly by inducing a conformational shift in the core; in agreement, HIV-1 cores bearing N74D regained their ability to bind CPSF6 in the presence of GS-CA1. We showed that GS-CA1 binds to the HIV-1 core, changes its conformation, stabilizes the core, and thereby prevents viral uncoating and infection.
Keywords: Biological sciences; Immunology; Virology.
© 2021.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

