Beat-to-beat dynamic regulation of intracellular pH in cardiomyocytes
- PMID: 35005560
- PMCID: PMC8718820
- DOI: 10.1016/j.isci.2021.103624
Beat-to-beat dynamic regulation of intracellular pH in cardiomyocytes
Abstract
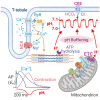
The mammalian heart beats incessantly with rhythmic mechanical activities generating acids that need to be buffered to maintain a stable intracellular pH (pHi) for normal cardiac function. Even though spatial pHi non-uniformity in cardiomyocytes has been documented, it remains unknown how pHi is regulated to match the dynamic cardiac contractions. Here, we demonstrated beat-to-beat intracellular acidification, termed pHi transients, in synchrony with cardiomyocyte contractions. The pHi transients are regulated by pacing rate, Cl-/HCO3 - transporters, pHi buffering capacity, and β-adrenergic signaling. Mitochondrial electron-transport chain inhibition attenuates the pHi transients, implicating mitochondrial activity in sculpting the pHi regulation. The pHi transients provide dynamic alterations of H+ transport required for ATP synthesis, and a decrease in pHi may serve as a negative feedback to cardiac contractions. Current findings dovetail with the prevailing three known dynamic systems, namely electrical, Ca2+, and mechanical systems, and may reveal broader features of pHi handling in excitable cells.
Keywords: Cardiovascular medicine; Molecular biology; Molecular dynamics.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Demonstration of Beat-to-Beat, On-Demand ATP Synthesis in Ventricular Myocytes Reveals Sex-Specific Mitochondrial and Cytosolic Dynamics.bioRxiv [Preprint]. 2025 Jul 10:2025.07.07.663572. doi: 10.1101/2025.07.07.663572. bioRxiv. 2025. PMID: 40672169 Free PMC article. Preprint.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
Cited by
-
Desmoplakin CSM models unravel mechanisms regulating the binding to intermediate filaments and putative therapeutics for cardiocutaneous diseases.Sci Rep. 2024 Oct 5;14(1):23206. doi: 10.1038/s41598-024-73705-0. Sci Rep. 2024. PMID: 39369039 Free PMC article.
-
Protocol to record and quantify the intracellular pH in contracting cardiomyocytes.STAR Protoc. 2022 Apr 12;3(2):101301. doi: 10.1016/j.xpro.2022.101301. eCollection 2022 Jun 17. STAR Protoc. 2022. PMID: 35463464 Free PMC article.
-
A novel method to extend viability and functionality of living heart slices.Front Cardiovasc Med. 2023 Oct 10;10:1244630. doi: 10.3389/fcvm.2023.1244630. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37881724 Free PMC article.
-
INNOVATIVE TECHNIQUES AND NEW INSIGHTS: Studying cardiac ionic currents and action potentials in physiologically relevant conditions.Physiol Mini Rev. 2023 May-Jun;16(3):22-34. Physiol Mini Rev. 2023. PMID: 38107545 Free PMC article.
-
STIM1 functions as a proton sensor to coordinate cytosolic pH with store-operated calcium entry.J Biol Chem. 2024 Dec;300(12):107924. doi: 10.1016/j.jbc.2024.107924. Epub 2024 Oct 23. J Biol Chem. 2024. PMID: 39454952 Free PMC article.
References
Grants and funding
- R01 HL123526/HL/NHLBI NIH HHS/United States
- I01 CX001490/CX/CSRD VA/United States
- R01 DC016099/DC/NIDCD NIH HHS/United States
- R01 DC015252/DC/NIDCD NIH HHS/United States
- R01 HL137228/HL/NHLBI NIH HHS/United States
- R01 HL085844/HL/NHLBI NIH HHS/United States
- T32 HL086350/HL/NHLBI NIH HHS/United States
- R01 HL081562/HL/NHLBI NIH HHS/United States
- R01 DC015135/DC/NIDCD NIH HHS/United States
- R01 HL170520/HL/NHLBI NIH HHS/United States
- R01 HL085727/HL/NHLBI NIH HHS/United States
- R01 AG060504/AG/NIA NIH HHS/United States
- R01 HL141460/HL/NHLBI NIH HHS/United States
- I01 BX000576/BX/BLRD VA/United States
- P01 AG051443/AG/NIA NIH HHS/United States
- R56 HL138392/HL/NHLBI NIH HHS/United States
- R01 HL159993/HL/NHLBI NIH HHS/United States
- F32 HL149288/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

