Anchoring the species Rhizophagus intraradices (formerly Glomus intraradices)
- PMID: 35005581
- PMCID: PMC8687058
- DOI: 10.3114/fuse.2021.08.14
Anchoring the species Rhizophagus intraradices (formerly Glomus intraradices)
Abstract
The nomenclatural type material of Rhizophagus intraradices (basionym Glomus intraradices) was originally described from a trap pot culture established with root fragments, subcultures of which later became registered in the INVAM culture collection as FL 208. Subcultures of FL 208 (designated as strain ATT 4) and a new strain, independently isolated from the type location (ATT 1102), were established as both pot cultures with soil-like substrate and in vitro root organ culture. Long-term sampling of these cultures shows spores of the species to have considerable morphological plasticity, not described in the original description. Size, shape and other features of the spores were much more variable than indicated in the protologue. Phylogenetic analyses confirmed earlier published evidence that sequences from all R. intraradices cultures formed a monophyletic clade, well separated from, and not representing a sister clade to, R. irregularis. Moreover, new phylogenetic analyses show that Rhizoglomus venetianum and R. irregularis are synonymous. The morphological characters used to separate these species exemplify the difficulties in species recognition due to the high phenotypic plasticity in the genus Rhizophagus. Rhizophagus intraradices is morphologically re-described, an epitype is designated from a single-spore isolate derived from ATT 4, and R. venetianum is synonymised with R. irregularis.
Keywords: Glomeromycota; arbuscular mycorrhizal fungi; molecular phylogeny; phenotypic plasticity; species definition.
© 2021 Westerdijk Fungal Biodiversity Institute.
Figures


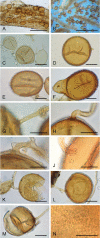






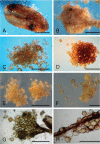
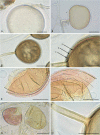


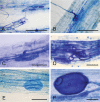
Similar articles
-
Synonymization of three species of Rhizophagus based on morphological and molecular evidence and biogeography of Rhizophagus clarus.Mycorrhiza. 2025 Feb 28;35(2):16. doi: 10.1007/s00572-025-01182-y. Mycorrhiza. 2025. PMID: 40019552
-
'Glomus intraradices DAOM197198', a model fungus in arbuscular mycorrhiza research, is not Glomus intraradices.New Phytol. 2009;183(4):1176-1187. doi: 10.1111/j.1469-8137.2009.02874.x. Epub 2009 Jun 3. New Phytol. 2009. PMID: 19496945
-
Characterization of arbuscular mycorrhizal fungal species associating with Zea mays.Front Plant Sci. 2024 May 7;15:1345229. doi: 10.3389/fpls.2024.1345229. eCollection 2024. Front Plant Sci. 2024. PMID: 38774223 Free PMC article.
-
Acaulospora brasiliensis comb. nov. and Acaulospora alpina (Glomeromycota) from upland Scotland: morphology, molecular phylogeny and DNA-based detection in roots.Mycorrhiza. 2011 Aug;21(6):577-587. doi: 10.1007/s00572-011-0361-7. Epub 2011 Feb 19. Mycorrhiza. 2011. PMID: 21336507
-
Growth dynamics of geographically different arbuscular mycorrhizal fungal isolates belonging to the 'Rhizophagus clade' under monoxenic conditions.Mycologia. 2014 Sep-Oct;106(5):963-75. doi: 10.3852/13-118. Epub 2014 Jun 2. Mycologia. 2014. PMID: 24891409
Cited by
-
The Metabolic Profile of Anchusa officinalis L. Differs According to Its Associated Arbuscular Mycorrhizal Fungi.Metabolites. 2022 Jun 22;12(7):573. doi: 10.3390/metabo12070573. Metabolites. 2022. PMID: 35888697 Free PMC article.
-
Influence of plant species, mycorrhizal inoculant, and soil phosphorus level on arbuscular mycorrhizal communities in onion and carrot roots.Front Plant Sci. 2024 Jan 15;14:1324626. doi: 10.3389/fpls.2023.1324626. eCollection 2023. Front Plant Sci. 2024. PMID: 38288412 Free PMC article.
-
Decoding Plant-Pathogen Interactions: A Comprehensive Exploration of Effector-Plant Transcription Factor Dynamics.Mol Plant Pathol. 2025 Jan;26(1):e70057. doi: 10.1111/mpp.70057. Mol Plant Pathol. 2025. PMID: 39854033 Free PMC article. Review.
-
Effect of arbuscular mycorrhizal symbiosis on growth and biochemical characteristics of Chinese fir (Cunninghamia lanceolata) seedlings under low phosphorus environment.PeerJ. 2024 Mar 22;12:e17138. doi: 10.7717/peerj.17138. eCollection 2024. PeerJ. 2024. PMID: 38529308 Free PMC article.
-
Spatial co-transcriptomics reveals discrete stages of the arbuscular mycorrhizal symbiosis.Nat Plants. 2024 Apr;10(4):673-688. doi: 10.1038/s41477-024-01666-3. Epub 2024 Apr 8. Nat Plants. 2024. PMID: 38589485 Free PMC article.
References
-
- Alaux P-L, Mison C, Senés-Guerrero, et al. (2021). Diversity and species composition of arbuscular mycorrhizal fungi across maize fields in the southern part of Belgium. Mycorrhiza 31: 265–272. - PubMed
-
- Anon (1969). Royal Botanic Garden Edinburgh, Flora of the British Fungi Colour Identification Chart. HMSO, Edinburgh, UK.
-
- Anon (1990). Munsell Soil Color Charts 1990 edition revised. Munsell Color Macbeth Division of Kollmorgen Instruments Corp., Maryland, USA.
-
- Błaszkowski J, Czerniawska B, Wubet T, et al. (2008). Glomus irregulare, a new arbuscular mycorrhizal fungus in the Glomeromycota. Mycotaxon 106: 247–267.
-
- Butler EJ. (1939). The occurrence and systematic position of the vesicular-arbuscular type of mycorrhizal fungi. Transactions of the British Mycological Society 22: 274–301
LinkOut - more resources
Full Text Sources
