Hypoxia represses early responses of prostate and renal cancer cells to YM155 independent of HIF-1α and HIF-2α
- PMID: 35005610
- PMCID: PMC8717246
- DOI: 10.1016/j.crphar.2021.100076
Hypoxia represses early responses of prostate and renal cancer cells to YM155 independent of HIF-1α and HIF-2α
Abstract
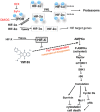
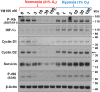
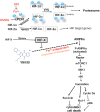
The imidazolium compound Sepantronium Bromide (YM155) successfully promotes tumor regression in various pre-clinical models but has shown modest responses in human clinical trials. We provide evidence to support that the hypoxic milieu of tumors may limit the clinical usefulness of YM155. Hypoxia (1% O2) strongly (>16-fold) represses the cytotoxic activity of YM155 on prostate and renal cancer cells in vitro. Hypoxia also represses all early signaling responses associated with YM155, including activation of AMPK and retinoblastoma protein (Rb), inactivation of the mechanistic target of rapamycin complex 1 (mTORC1), inhibition of phospho-ribosomal protein S6 (rS6), and suppression of the expression of Cyclin Ds, Mcl-1 and Survivin. Cells pre-incubated with hypoxia for 24 h are desensitized to YM155 even when they are treated with YM155 under atmospheric oxygen conditions, supporting that cells at least temporarily retain hypoxia-induced resistance to YM155. We tested the role of hypoxia-inducible factor (HIF)-1α and HIF-2α in the hypoxia-induced resistance to YM155 by comparing responses of YM155 in VHL-proficient versus VHL-deficient RCC4 and 786-O renal cancer cells and silencing HIF expression in PC-3 prostate cancer cells. Those studies suggested that hypoxia-induced resistance to YM155 occurs independent of HIF-1α and HIF-2α. Moreover, the hypoxia mimetics deferoxamine and dimethyloxalylglycine, which robustly induce HIF-1α levels in PC-3 cells under atmospheric oxygen, did not diminish their early cellular responses to YM155. Collectively, our data support that hypoxia induces resistance of cells to YM155 through a HIF-1α and HIF-2α-independent mechanism. We hypothesize that a hypothetical hypoxia-inducer factor (HIF-X) represses early signaling responses to YM155.
Keywords: AMPK; Drug resistance; Genitourinary cancers; Hypoxia; VHL; mTORC1.
© 2021 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: David Danielpour reports was provided by Case Western Reserve University. David Danielpour reports a relationship with Case Western Reserve University that includes:. David Danielpour has patent pending to none. none.
Figures





Similar articles
-
Early Cellular Responses of Prostate Carcinoma Cells to Sepantronium Bromide (YM155) Involve Suppression of mTORC1 by AMPK.Sci Rep. 2019 Aug 8;9(1):11541. doi: 10.1038/s41598-019-47573-y. Sci Rep. 2019. PMID: 31395901 Free PMC article.
-
Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function.Oncogene. 2000 Nov 16;19(48):5435-43. doi: 10.1038/sj.onc.1203938. Oncogene. 2000. PMID: 11114720
-
Lenticular cytoprotection. Part 1: the role of hypoxia inducible factors-1α and -2α and vascular endothelial growth factor in lens epithelial cell survival in hypoxia.Mol Vis. 2013;19:1-15. Epub 2013 Jan 2. Mol Vis. 2013. PMID: 23335846 Free PMC article.
-
Suppressive effects of iron chelation in clear cell renal cell carcinoma and their dependency on VHL inactivation.Free Radic Biol Med. 2019 Mar;133:295-309. doi: 10.1016/j.freeradbiomed.2018.12.013. Epub 2018 Dec 13. Free Radic Biol Med. 2019. PMID: 30553971 Free PMC article.
-
Mutual Antagonism of Hypoxia-Inducible Factor Isoforms in Cardiac, Vascular, and Renal Disorders.JACC Basic Transl Sci. 2020 Sep 28;5(9):961-968. doi: 10.1016/j.jacbts.2020.05.006. eCollection 2020 Sep. JACC Basic Transl Sci. 2020. PMID: 33015417 Free PMC article. Review.
Cited by
-
Jagged-1 is induced by mTOR inhibitors in renal cancer cells through an Akt/ALK5/Smad4-dependent mechanism.Curr Res Pharmacol Drug Discov. 2022 Jul 4;3:100117. doi: 10.1016/j.crphar.2022.100117. eCollection 2022. Curr Res Pharmacol Drug Discov. 2022. PMID: 35992379 Free PMC article.
References
-
- Aoyama Y., Kaibara A., Takada A., Nishimura T., Katashima M., Sawamoto T. Population pharmacokinetic modeling of sepantronium bromide (YM155), a small molecule survivin suppressant, in patients with non-small cell lung cancer, hormone refractory prostate cancer, or unresectable stage III or IV melanoma. Invest. N. Drugs. 2013;31:443–451. - PMC - PubMed
-
- Baek J.H., Jang J.E., Kang C.M., Chung H.Y., Kim N.D., Kim K.W. Hypoxia-induced VEGF enhances tumor survivability via suppression of serum deprivation-induced apoptosis. Oncogene. 2000;19:4621–4631. - PubMed
-
- Baspinar Y., Erel-Akbaba G., Kotmakci M., Akbaba H. Development and characterization of nanobubbles containing paclitaxel and survivin inhibitor YM155 against lung cancer. Int. J. Pharm. 2019;566:149–156. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

