HLA-A∗03:01 is associated with increased risk of fever, chills, and stronger side effects from Pfizer-BioNTech COVID-19 vaccination
- PMID: 35005651
- PMCID: PMC8719913
- DOI: 10.1016/j.xhgg.2021.100084
HLA-A∗03:01 is associated with increased risk of fever, chills, and stronger side effects from Pfizer-BioNTech COVID-19 vaccination
Abstract
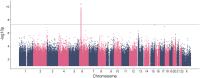
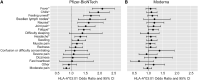
COVID-19 vaccines are safe and highly effective, but some individuals experience unpleasant reactions to vaccination. As the majority of adults in the United States have received a COVID-19 vaccine this year, there is an unprecedented opportunity to study the genetics of reactions to vaccination via surveys of individuals who are already part of genetic research studies. Here, we have queried 17,440 participants in the Helix DNA Discovery Project and Healthy Nevada Project about their reactions to COVID-19 vaccination. Our genome-wide association study identifies an association between severe difficulties with daily routine after vaccination and HLA-A∗03:01. This association was statistically significant only for those who received the Pfizer-BioNTech vaccine (BNT162b2; n = 3,694; p = 4.70E-11; OR = 2.07 [95% CI 1.67-2.56]), and showed a smaller effect size in those who received the Moderna vaccine (mRNA-1273; n = 3,610; p = 0.005; OR = 1.32 [95% CI 1.09-1.59]). In Pfizer-BioNTech recipients, HLA-A∗03:01 was associated with a 2-fold increase in risk of self-reported severe difficulties with daily routine following vaccination. The effect was consistent across ages, sexes, and whether the person had previously had a COVID-19 infection. The reactions experienced by HLA-A∗03:01 carriers were driven by associations with chills, fever, fatigue, and generally feeling unwell.
Keywords: COVID; COVID-19; GWAS; HLA; HLA-A; HLA-A∗03:01; SARS-CoV-2; vaccination.
© 2021 The Author(s).
Conflict of interest statement
A.B., K.M.S.B., S.W., M.I., W.L., N.L.W., and E.T.C. are employees of Helix.
Figures

References
-
- Chapin-Bardales J., Gee J., Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. 2021;325:2201–2202. - PubMed
-
- Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., Sudre C.H., Nguyen L.H., Drew D.A., Merino J., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect. Dis. 2021;21:939–949. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

