Insecticide-impregnated netting: A surface treatment for killing Lutzomyia longipalpis (Diptera: Psychodidae), the vector of Leishmania infantum
- PMID: 35005688
- PMCID: PMC8716342
- DOI: 10.1016/j.crpvbd.2021.100044
Insecticide-impregnated netting: A surface treatment for killing Lutzomyia longipalpis (Diptera: Psychodidae), the vector of Leishmania infantum
Abstract
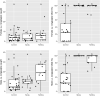
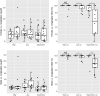
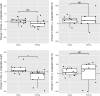
The sand fly Lutzomyia longipalpis is the main vector of Leishmania infantum in Brazil. Synthetic male-produced sex/aggregation pheromone co-located with micro-encapsulated λ-cyhalothrin in chicken sheds can significantly reduce canine infection and sand fly densities in a lure-and-kill strategy. In this study, we determined if insecticide-impregnated netting (IN) could replace insecticide residual spraying (IRS). We compared numbers of Lu. longipalpis attracted and killed in experimental and real chicken sheds baited with pheromone and treated with a 1 m2 area of either insecticide spray or netting. First, we compared both treatments in experimental sheds to control mortality established from light trap captures. We then compared the long-term killing effect of insecticide spray and netting, without renewal, in experimental sheds over a period of 16 weeks. Finally, a longitudinal intervention study in real chicken sheds compared the numbers and proportions of Lu. longipalpis collected and killed before and after application of both treatments. In Experiment 1, a higher proportion of males and females captured in IRS- and IN-treated sheds were dead at 24 h compared to controls (P < 0.05). No difference was found in the proportion of females killed in sheds treated with IN or IRS (P = 0.15). A slightly higher proportion of males were killed by IRS (100%) compared to IN (98.6%; P < 0.05). In Experiment 2, IN- and IRS-treated traps were equally effective at killing females (P = 0.21) and males (P = 0.08). However, IRS killed a significantly higher proportion of females and males after 8 (P < 0.05) and 16 (P < 0.05) weeks. In Experiment 3, there was no significant difference between treatments in the proportion of females killed before (P = 0.88) or after (P = 0.29) or males killed before (P = 0.76) or after (P = 0.73) intervention. Overall, initially the IN was as effective as IRS at killing female and male Lu. longipalpis in both experimental and real chicken sheds. However, the relative lethal effect of the IN deteriorated over time when stored under prevailing environmental conditions.
Keywords: (±)-9-methylgermacrene-B; Interceptor; Leishmania infantum; Lutzomyia longipalpis; Olyset plus; Sex-aggregation pheromone; α-cypermethrin; λ-cyhalothrin.
© 2021 The Author(s).
Figures


References
-
- Alexander B., Barros V.C., De Souza S.F., Barros S.S., Teodoro L.P., Soares Z.R., et al. Susceptibility to chemical insecticides of two Brazilian populations of the visceral leishmaniasis vector Lutzomyia longipalpis (Diptera: Psychodidae) Trop. Med. Int. Health. 2009;14:1272–1277. - PubMed
-
- Alexander B., Usma M.C., Cadena H., Quesada B.L., Solarte Y., Roa W., Travi B.L. Evaluation of deltamethrin-impregnated bednets and curtains against phlebotomine sandflies in Valle del Cauca, Colombia. Med. Vet. Entomol. 1995;9:279–283. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

