Treatment for recurrent vulvovaginal candidiasis (thrush)
- PMID: 35005777
- PMCID: PMC8744138
- DOI: 10.1002/14651858.CD009151.pub2
Treatment for recurrent vulvovaginal candidiasis (thrush)
Abstract
Background: Recurrent vulvovaginal candidiasis (RVVC) affects up to 5% of women. No comprehensive systematic review of treatments for RVVC has been published.
Objectives: The primary objective was to assess the effectiveness and safety of pharmacological and non-pharmacological treatments for RVVC. The secondary objective was to assess patient preference of treatment options.
Search methods: We conducted electronic searches of bibliographic databases, including CENTRAL, MEDLINE, Embase, and CINAHL (search date 6 October 2021). We also handsearched reference lists of identified trials and contacted authors of identified trials, experts in RVVC, and manufacturers of products for vulvovaginal candidiasis.
Selection criteria: We considered all published and unpublished randomised controlled trials evaluating RVVC treatments for at least six months, in women with four or more symptomatic episodes of vulvovaginal candidiasis in the past year. We excluded women with immunosuppressive disorders or taking immunosuppressant medication. We included women with diabetes mellitus and pregnant women. Diagnosis of RVVC must have been confirmed by presence of symptoms and a positive culture and/or microscopy. We included all drug and non-drug therapies and partner treatment, assessing the following primary outcomes: • number of clinical recurrences per participant per year (recurrence defined as clinical signs and positive culture/microscopy); • proportion of participants with at least one clinical recurrence during the treatment and follow-up period; and • adverse events.
Data collection and analysis: Two authors independently reviewed titles and abstracts to identify eligible trials. Duplicate data extraction was completed independently by two authors. We assessed risk of bias as described in the Cochrane Handbook for Systematic Reviews of Interventions. We used the fixed-effects model for pooling and expressed the results as risk ratio (RR) with 95% confidence intervals (CI). Where important statistical heterogeneity was present we either did not pool data (I2 > 70%) or used a random-effects model (I2 40-70%). We used the GRADE tool to assess overall certainty of the evidence for the pooled primary outcomes.
Main results: Studies: Twenty-three studies involving 2212 women aged 17 to 67 years met the inclusion criteria. Most studies excluded pregnant women and women with diabetes or immunosuppression. The predominant species found on culture at study entry was Candida albicans. Overall, the included studies were small (<100 participants). Six studies compared antifungal treatment with placebo (607 participants); four studies compared oral versus topical antifungals (543 participants); one study compared different oral antifungals (45 participants); two studies compared different dosing regimens for antifungals (100 participants); one study compared two different dosing regimens of the same topical agent (23 participants); one study compared short versus longer treatment duration (26 participants); two studies assessed the effect of partner treatment (98 participants); one study compared a complementary treatment (Lactobacillus vaginal tablets and probiotic oral tablets) with placebo (34 participants); three studies compared complementary medicine with antifungals (354 participants); two studies compared 'dermasilk' briefs with cotton briefs (130 participants); one study examined Lactobacillus vaccination versus heliotherapy versus ciclopyroxolamine (90 participants); one study compared CAM treatments to an antifungal treatment combined with CAM treatments (68 participants). We did not find any studies comparing different topical antifungals. Nine studies reported industry funding, three were funded by an independent source and eleven did not report their funding source. Risk of bias: Overall, the risk of bias was high or unclear due to insufficient blinding of allocation and participants and poor reporting. Primary outcomes: Meta-analyses comparing drug treatments (oral and topical) with placebo or no treatment showed there may be a clinically relevant reduction in clinical recurrence at 6 months (RR 0.36, 95% CI 0.21 to 0.63; number needed to treat for an additional beneficial outcome (NNTB) = 2; participants = 607; studies = 6; I² = 82%; low-certainty evidence) and 12 months (RR 0.80, 95% CI 0.72 to 0.89; NNTB = 6; participants = 585; studies = 6; I² = 21%; low-certainty evidence). No study reported on the number of clinical recurrences per participant per year. We are very uncertain whether oral drug treatment compared to topical treatment increases the risk of clinical recurrence at 6 months (RR 1.66, 95% CI 0.83 to 3.31; participants = 206; studies = 3; I² = 0%; very low-certainty evidence) and reduces the risk of clinical recurrence at 12 months (RR 0.95, 95% CI 0.71 to 1.27; participants = 206; studies = 3; I² = 10%; very low-certainty evidence). No study reported on the number of clinical recurrences per participant per year. Adverse events were scarce across both treatment and control groups in both comparisons. The reporting of adverse events varied amongst studies, was generally of very low quality and could not be pooled. Overall the adverse event rate was low for both placebo and treatment arms and ranged from less than 5% to no side effects or complications.
Authors' conclusions: In women with RVVC, treatment with oral or topical antifungals may reduce symptomatic clinical recurrences when compared to placebo or no treatment. We were unable to find clear differences between different treatment options (e.g. oral versus topical treatment, different doses and durations). These findings are not applicable to pregnant or immunocompromised women and women with diabetes as the studies did not include or report on them. More research is needed to determine the optimal medication, dose and frequency.
Trial registration: ClinicalTrials.gov NCT00915629.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Georga Cooke: no conflicts of interest Cathy Watson: no conflicts of interest Laura Deckx: no conflicts of interest Marie Pirotta: no conflicts of interest Jane Smith: no conflicts of interest Mieke L van Driel: no conflicts of interest
Figures
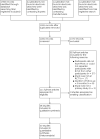

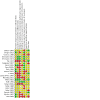



























Update of
- doi: 10.1002/14651858.CD009151
Similar articles
-
Topical clonidine for neuropathic pain in adults.Cochrane Database Syst Rev. 2022 May 19;5(5):CD010967. doi: 10.1002/14651858.CD010967.pub3. Cochrane Database Syst Rev. 2022. PMID: 35587172 Free PMC article.
-
Acupuncture for treating overactive bladder in adults.Cochrane Database Syst Rev. 2022 Sep 23;9(9):CD013519. doi: 10.1002/14651858.CD013519.pub2. Cochrane Database Syst Rev. 2022. PMID: 36148895 Free PMC article.
-
Interventions for infantile haemangiomas of the skin.Cochrane Database Syst Rev. 2018 Apr 18;4(4):CD006545. doi: 10.1002/14651858.CD006545.pub3. Cochrane Database Syst Rev. 2018. PMID: 29667726 Free PMC article.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2021 Sep 14;9(9):CD010216. doi: 10.1002/14651858.CD010216.pub6. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD010216. doi: 10.1002/14651858.CD010216.pub7. PMID: 34519354 Free PMC article. Updated.
-
Oral antifungal medication for toenail onychomycosis.Cochrane Database Syst Rev. 2017 Jul 14;7(7):CD010031. doi: 10.1002/14651858.CD010031.pub2. Cochrane Database Syst Rev. 2017. PMID: 28707751 Free PMC article.
Cited by
-
Intravaginal Combination Therapy of Acetic and Lactic Acid in premenopausal women with recurrent vulvovaginal candidiasis: A randomized, double-blind placebo-controlled feasibility trial.Womens Health (Lond). 2023 Jan-Dec;19:17455057231194138. doi: 10.1177/17455057231194138. Womens Health (Lond). 2023. PMID: 37635435 Free PMC article. Clinical Trial.
-
Inhibitory effects of vaginal Lactobacilli on Candida albicans growth, hyphal formation, biofilm development, and epithelial cell adhesion.Front Cell Infect Microbiol. 2023 May 2;13:1113401. doi: 10.3389/fcimb.2023.1113401. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37201113 Free PMC article.
-
Effects of Lactiplantibacillus plantarum LM1215 on Candida albicans and Gardnerella vaginalis.Yonsei Med J. 2024 Dec;65(12):727-740. doi: 10.3349/ymj.2023.0490. Yonsei Med J. 2024. PMID: 39609088 Free PMC article.
-
The Increasing Trend of Triazole-Resistant Candida from Vulvovaginal Candidiasis.Infect Drug Resist. 2024 Oct 5;17:4301-4310. doi: 10.2147/IDR.S474304. eCollection 2024. Infect Drug Resist. 2024. PMID: 39385847 Free PMC article.
-
Efficacy of Limosilactobacillus fermentum in the management of vulvovaginal candidiasis: comparative analysis with topical miconazole in a single-blind randomized clinical trial.Front Microbiol. 2024 Aug 1;15:1428590. doi: 10.3389/fmicb.2024.1428590. eCollection 2024. Front Microbiol. 2024. PMID: 39149209 Free PMC article.
References
References to studies included in this review
Bolouri 2009 {published data only (unpublished sought but not used)}
-
- Bolouri F, Tabrizi NM, Tanha FD, Niroomand N, Azmoodeh A, Emami S, et al.Effectiveness of fluconazole for suppressive maintenance therapy in patients with RVVC: a randomized placebo-controlled study. Iranian Journal of Pharmaceutical Research 2009;8:307-13.
Chopra 2013 {published and unpublished data}
-
- Chopra V, Marotta F, Kumari A, Bishier MP, He F, Zerbinati N, et al.Prophylactic strategies in recurrent vulvovaginal candidiasis: a 2-year study testing a phytonutrient vs itraconazole. Journal of Biological Regulators and Homeostatic Agents 2013;27(3):875-82. [PMID: ] - PubMed
Corthay 1988 {published data only (unpublished sought but not used)}
-
- Corthay P, Perrenoud C, Gumowski PI, Girard JP.Candida albicans specific immune responses in patients with recurrent chronic vaginitis treated with an anti-candida food regimen (ACFR) or an hyposensitization treatment (HPT). Allergy 1988;43:51.
D'Antuono 2012 {published and unpublished data}
-
- D'Antuono A, Baldi E, Bellavista S, Banzola N, Zauli S, Patrizi A.Use of Dermasilk briefs in recurrent vulvovaginal candidosis: safety and effectiveness. Mycoses 2012;55(3):e85-9. - PubMed
D'Antuono 2013 {published data only (unpublished sought but not used)}
-
- D'Antuono A, Bellavista S, Gaspari V, Filippini A, Patrizi A.Dermasilk® briefs in recurrent vulvovaginal candidosis. An alternative option in long-lasting disease. Minerva Ginecologica 2013;65(6):697-705. - PubMed
Fan 2015 {published data only}
-
- Fan S, Liu X, Wu C, Xu L, Li J.Vaginal nystatin versus oral fluconazole for the treatment for recurrent vulvovaginal candidiasis. Mycopathologia 2015;179:95-101. [DOI: ] - PubMed
Fardyazar 2007 {published data only (unpublished sought but not used)}
-
- Fardyazar Z, Habibzadeh S, Abdollahi-Fard S, Tello M.Vaginal azoles versus oral fluconazole in treatment of recurrent vulvovaginal candidiasis. Iranian Journal of Clinical Infectious Diseases 2007;2:17-22.
-
- Fardyazar Z, Habibzadeh S.Long term vaginal azoles versus prophylactic oral fluconazole in treatment of recurrent vulvovaginal candidiasis. Research Journal of Biological Sciences 2007;2(6):663-6.
Fong 1992a {published data only (unpublished sought but not used)}
Fong 1992b {published data only (unpublished sought but not used)}
Fong 1994 {published data only}
Kumari 2011 {published data only}
-
- Kumari A, Bishier MP, Naito Y, Sharma A, Solimene U, Jain S, et al.Protective effect of an oral natural phytonutrient in recurrent vulvovaginal candidiasis: a 12-month study. Journal of Biological Regulators & Homeostatic Agents 2011;25(4):543-51. - PubMed
Li 2018 {published data only}
Lopez‐Olmos 2000 {published and unpublished data}
-
- Lopez-Olmos J, Lerma E.Treatment of recurring vulvo-vaginal candidiasis: a comparative prospective study during six months of three anti-mycotic preparations of a single dosis. Clinica e Investigacion en Ginecologia y Obstetricia 2000;27:366-75.
Mendling 2011 {published data only}
-
- Mendling W, Birkner V.Vaccination with inactivated lactobacilli or heliotherapy can improve the quality-of-life of women with chronic recurrent vulvovaginal candidosis a prospective randomized study [Die Vakzination mit inaktivierten Laktobazillen oder mit Heliotherapie kann die Lebensqualitat von Frauen mit rezidivierender vulvovaginaler Kandidose verbessern - Eine prospektive 3-armige randomisierte Studie]. Geburtshilfe und Frauenheilkunde 2011;71(9):767-72.
Metts 2003 {published data only (unpublished sought but not used)}
-
- Metts J, Famula TR, Trenev N, Clemens RA.Lactobacillus acidophilus, strain NAS (H2O2 positive), in reduction of recurrent candidal vulvovaginitis. Journal of Applied Research 2003;3:340-8.
Roth 1990 {published data only (unpublished sought but not used)}
Sobel 1985a {published data only (unpublished sought but not used)}
-
- Sobel JD.Management of recurrent vulvovaginal candidiasis (VVC) with ketoconazole (K) prophylaxis [abstract]. Archives of Gynecology 1985;237 Suppl:281.
-
- Sobel JD.Management of recurrent vulvovaginal candidiasis with intermittent ketoconazole prophylaxis. Obstetrics & Gynecology 1985;65:435-40. - PubMed
Sobel 1986 {published data only (unpublished sought but not used)}
-
- Sobel JD.Recurrent vulvovaginal candidiasis. A prospective study of the efficacy of maintenance ketoconazole therapy. New England Journal of Medicine 1986;315:1455-8. - PubMed
Sobel 1989 {published data only (unpublished sought but not used)}
-
- Sobel JD, Schmitt C, Meriwether C.Clotrimazole treatment of recurrent and chronic candida vulvovaginitis. Obstetrics & Gynecology 1989;73:330-4. - PubMed
Sobel 2004 {published data only (unpublished sought but not used)}
-
- Sobel JD, Wiesenfeld HC, Martens M, Danna P, Hooton TM, Rompalo A, et al.Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. New England Journal of Medicine 2004;351:876-83. - PubMed
Spacek 2005 {published data only}
-
- Spacek J, Buchta V.Itraconazole in the treatment of acute and recurrent vulvovaginal candidosis: comparison of a 1-day and a 3-day regimen. Mycoses 2005;48:165-71. - PubMed
Spinillo 1997 {published data only}
-
- Spinillo A, Colonna L, Piazzi G, Baltaro F, Monaco A, Ferrari A.Managing recurrent vulvovaginal candidiasis. Intermittent prevention with itraconazole. Journal of Reproductive Medicine 1997;42:83-7. - PubMed
Witt 2009 {published data only (unpublished sought but not used)}
-
- Witt A, Kaufmann U, Bitschnau M, Tempfer C, Ozbal A, Haytouglu E, et al.Monthly itraconazole versus classic homeopathy for the treatment of recurrent vulvovaginal candidiasis: a randomized trial. Obstetrical and Gynecological Survey 2010;65(1):26-7. - PubMed
-
- Witt A, Kaufmann U, Bitschnau M, Tempfer C, Ozbal A, Haytouglu E, Gregor H, Kiss H.Monthly itraconazole versus classic homeopathy for the treatment of recurrent vulvovaginal candidiasis: a randomised trial. British Journal of Obstetrics & Gynaecology 2009;116(11):1499-505. - PubMed
References to studies excluded from this review
Avijgan 2012 {published data only}
-
- Avijgan M, Mirzadeh F, Nia EA.The comparative study of anti-fungal effect of pharmaceutical products containing hydroalcoholic extract of Echinophora platyloba DC and fluconazole in women with chronic recurrent vaginitis caused by candida albicans. Journal of Research in Medical Sciences 2012;17:S103-S107.
Azima 2018 {published data only}
-
- Azima S, Houshyar S, Motamedi Far M, Kaviani M, Zare N.The Effect of Vaginal Probiotic Capsule On Vaginal Colonization and Treatment Results inPatients with Vulvar and Vaginal Candidiasis. Journal of Zanjan University of Medical Sciences and Health Services 2018;26(114):40-50.
Bartz 2000 {published data only}
-
- Bartz BR.Efficacy of zinc supplementation in reducing the incidence of recurrent vaginitis. ProQuest Dissertations and Theses 2000:64.
Brand 2018 {published data only}
-
- Brand S, Degenhardt T, Nyirjesy P, Sobel J, Handelsman C, Person K, Schotzinger R, Tavakkol A.To evaluate the efficacy and safety of VT-1161, a potent, highly selective inhibitor of fungal CYP51, in treating women with a documented history of recurrent vulvovaginal candidiasis (RVVC). American Journal of Obstetrics and Gynecology 2016;215(6):S821. [DOI: 10.1016/j.ajog.2016.09.016] - DOI
-
- Brand SR, Degenhardt TP, Person K, Sobel JD, Nyiriesy P, Schotzinger RJ, Tavakkol A.A phase 2, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of orally administered VT-1161 in the treatment of recurrent vulvovaginal candidiasis. American Journal of Obstetrics and Gynecology 2018;218(6):1e.1-1e.9. - PubMed
-
- Sobel JD, Brand SR, Degenhardt TP, Person K, Nyirjesy P, Schotzinger RJ, Tavakkol A.Results from a phase 2, randomized, double-blind, placebo-controlled, dose ranging study to evaluate the efficacy and safety of VT 1161 oral tablets in the treatment of patients with recurrent vulvovaginal candidiasis. American Journal of Obstetrics and Gynecology 2017;217(6):715. - PubMed
Bushell 1988a {published data only}
Bushell 1998b {published data only}
-
- Bushell TE, Evans EG, Llewellyn PA, Meaden JD, Milne JD, Warnock DW.Prophylactic use of clotrimazole in recurrent vaginal candidosis. Annals of the New York Academy of Sciences 1988;544:558-60. [ISSN 0077-8923] - PubMed
Coric 2006 {published data only}
-
- Coric M, Barisic D, Lovric H.Fluconazole versus 3-day clotrimazole in the treatment of sporadic and recurrent vulvovaginal candidiasis. International Journal of Gynaecology & Obstetrics 2006;95:171-2. - PubMed
Davidson 1978 {published data only}
Diba 2010 {published data only}
-
- Diba K, Namaki A, Bahadori F.Comparative study of fluconazole and clotrimazole for the treatment of vulvovaginal candidiasis. Journal of Sexual Medicine 2010;7:431.
Donders 2008 {published data only}
-
- Donders G, Bellen G, Byttebier G, Verguts L, Hinoul P, Walckiers R, Stalpaert M, Vereecken A, Van Eldere J.Individualized decreasing-dose maintenance fluconazole regimen for recurrent vulvovaginal candidiasis (ReCiDiF trial). American Journal of Obstetrics & Gynecology 2008;199(6):613.e1-9. [ISSN 1097-6868] - PubMed
Edwards 2018 {published data only}
-
- Nyirjesy P, Sobel JD, Edwards JE, Schwarz MW, Schmidt CS, Hennessey JP.NDV-3A vaccine reduces the frequency of vaginitis in patients with recurrent vulvovaginal candidiasis. American Journal of Obstetrics and Gynecology 2016;215(6):S822. [DOI: 10.1016/j.ajog.2016.09.019] - DOI
Fan 2005 {published data only}
-
- Fan SR, Liu XP, Li JW, Xu AP, Liu M.Study on classification and treatment of vulvovaginal candidiasis. Chung-Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2005;40:532-5. - PubMed
Fong 1993 {published data only (unpublished sought but not used)}
Golero 1993 {published data only}
-
- Gorlero F, Larosa E, Cauwenbergh G, Woestenborghs R, Heykants J, Cilli P, Dececco L.Itraconazole plasma and vaginal mucosal levels in patients with chronic vaginal candidosis treated with itraconazole 200mg once-daily for 3 consecutive days. Drug Investigation 1993;6(1):22-24. [ISSN 0114-2402]
Hilton 1992 {published data only (unpublished sought but not used)}
-
- Hilton E, Isenberg HD, Alperstein P, France K, Borenstein MT.Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Annals of Internal Medicine 1992;116:353-7. - PubMed
Houang 1989 {published data only}
-
- Houang E.Fluconazole in recurrent vaginal candidiasis - preliminary-results of a double-blind, randomized clinical-trial. In: Fluconazole and its role in vaginal candidiasis. In: Richardson RG, editors(s). International Congress and Symposium Series. Vol. 160. London: Royal Society of Medicine Services, 1989:22-31.
Miller 1984 {published data only}
-
- Miller PI, Humphries M, Grassick K.A single-blind comparison of oral and intravaginal treatments in acute and recurrent vaginal candidosis in general practice. Pharmatherapeutica 1984;3:582-7. - PubMed
Rashid 1991 {published data only}
Shalev 1996 {published data only}
-
- Shalev E, Battino S, Weiner E, Colodner R, Keness Y.Ingestion of yogurt containing Lactobacillus acidophilus compared with pasteurized yogurt as prophylaxis for recurrent candidal vaginitis and bacterial vaginosis. Archives of Family Medicine 1996;5:593-6. - PubMed
Silverman 1971 {published data only}
-
- Silverman M, Okun R.Oxytetracycline-nystatin in the prevention of candidal vaginitis. American Journal of Obstetrics & Gynecology 1971;111:398-404. - PubMed
Sobel 1994 {published data only}
-
- Sobel JD, Schmitt C, Stein G, Mummaw N, Christensen S, Meriwether C.Initial management of recurrent vulvovaginal candidiasis with oral ketoconazole and topical clotrimazole. Journal of Reproductive Medicine 1994;39(7):517-20. [ISSN 0024-7758] - PubMed
Sobel 2001 {published data only}
-
- Sobel JD, Kapernick PS, Zervos M, Reed BD, Hooton T, Soper D, et al.Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. American Journal of Obstetrics & Gynecology 2001;185:363-9. - PubMed
Vladareanu 2018 {published data only}
-
- Vladareanu R, Mihu D, Mitran M, Mehedintu C, Boiangiu A, Manolache M, Vladareanu S.New evidence on oral L. plantarum P17630 product in women with history of recurrent vulvovaginal candidiasis (RVVC): a randomized double-blind placebo-controlled study. European Review for Medical and Pharmacological Sciences 2018;22(1):262-267. [DOI: 10.26355/eurrev_201801_14128] - DOI - PubMed
Yang 2000 {published data only}
-
- Yang F, Zhou H, Mo XZ, Yan DD.Itraconazole maintaining therapy in prevention recurrent vulva-vaginal candidiasis (Chinese). Chinese Journal of Leprosy and Skin Disease 2000;16(4):235.
References to studies awaiting assessment
ACTRN12614001258640 {published data only (unpublished sought but not used)}
-
- ACTRN12614001258640.A randomized, double-blinded study investigating the safety and efficacy of daily low-dose oral fluconazole versus weekly fluconazole in patients with chronic vulvovaginal candidiasis. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=1261... 2 December 2012.
ChiCTR‐IPR‐15006314 {published data only (unpublished sought but not used)}
-
- ChiCTR-IPR-15006314.Multi-center clinical study on JUC Spray Dressing under the patent technology "Physical Antimicrobial Film" in preventing the recurrence of vulvovaginal candidiasis (VVC). http://www.chictr.org.cn/showproj.aspx?proj=10772 14 April 2015.
ChiCTR‐TRC‐10000833 {published data only (unpublished sought but not used)}
-
- ChiCTR-TRC-10000833.The clinical research of Chinese herb XiangLian Suppository in the treatment of recurrent vulvovaginal candidiasis. http://www.chictr.org.cn/showproj.aspx?proj=8703 28 March 2010.
EUCTR2010‐021502‐38‐DE {published data only}
-
- EUCTR2010-021502-38-DE.Study for measuring vaginal interleukins in patients with cervical neoplasia (CIN 1-3) or chronic vaginal candidiasis with phenyl-4-butyrate [STUDIE ZUR MESSUNG DER VAGINALEN INTERLEUKINE BEI PATIENTINNEN MIT ZERVIKALEN NEOPLASIEN (CIN 1-3) ODER CHRONISCHER CANDIDIASIS VAGINALIS UNTER BEHANDLUNG MIT 4PHENYL-BUTYRATE]. https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-021502-38/DE 11 November 2010. [2010-021502-38]
EUCTR2011‐004718‐40‐IT {published data only}
-
- EUCTR2011-004718-40-IT.Activities of Metronidazole + Clotrimazole in the treatment and prophylaxis of recurrent vaginal infections recurrent Candida albicans and Candida albicans spp non albicans. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_nu... 8 March 2012. [2011-004718-40]
EUCTR2013‐002480‐26‐PL {published data only (unpublished sought but not used)}
-
- EUCTR2013-002480-26-PL.Study of the efficacy and safety of treatment with total freeze-dried culture of Lcr Regenerans® administered intravaginally in the prevention of recurrent vulvovaginal candidiasis.International, randomized, phase III, multi-centre, 2-arm, parallel group, double-blind, placebo-controlled superiority trial.. https://www.clinicaltrialsregister.eu/ctr-search/trial/2013-002480-26/PL 30 July 2014. [EUCTR2013-002480-26-PL]
NCT00479947 {published data only (unpublished sought but not used)}
-
- NCT00479947.Use of Oral Probiotics as an Adjunctive Therapy to Fluconazole in the Treatment of Yeast Vaginitis. http://clinicaltrials.gov/show/NCT00479947 30 May 2007. [NCT00479947]
NCT00915629 {published data only}
-
- NCT00915629.Prevention of Recurrent Vulvovaginal Candidiasis With Lactibiane Candisis 5M®. https://clinicaltrials.gov/ct2/show/NCT00915629 8 June 2009.
NCT02251093 {published data only (unpublished sought but not used)}
-
- NCT02251093.Study of the Efficacy and Tolerance of Intra-vaginal Treatment With a Total Freeze-dried Culture of Lcr Regenerans® in the Prevention of Relapses of Recurrent Vulvovaginal Candidiasis. https://clinicaltrials.gov/ct2/show/NCT02251093 26 September 2014.
Rabiee 2013 {published data only}
-
- IRCT2012102911302N1.Comparison of clinical response to vaginal cream zataria multiflora and boric acid in patients with recurrent vulvovaginal candidiasis. http://en.irct.ir/trial/11581 17 December 2012. [IRCT2012102911302N1]
-
- Rabiee M, Akbari H, Naseri M, Bekhradi R, Nikzad M, Torkestani F.Comparison of clinical response to Zatariamultiflora vaginal cream and boric acid inpatients with recurrent vulvovaginal candidiasis. Scientific-ResearchJournal of Shahed University 2013;102:1-8.
RBR‐892mp4 {published data only (unpublished sought but not used)}
-
- lRBR-892mp4.Effectiveness of therapeutic exercise applied to the pelvic floor muscles to prevent recurrence of Vulvovaginitis and improve female sexuality: randomized clinical trial. http://www.ensaiosclinicos.gov.br/rg/RBR-892mp4/ 17 June 2015. [RBR-892mp4]
Russo 2019 {published data only}
-
- Russo R, Superti F, Karadja E, De Seta F.Randomised clinical trial in women with Recurrent Vulvovaginal Candidiasis: Efficacy of probiotics and lactoferrin as maintenance treatment. Mycoses 2019;62:328–335. - PubMed
Zivaljevic 2012 {published data only}
-
- Zivaljevic B, Golubovic I, Seratlic J, Nikolic P, Simic D, Magdic I, et al.Efficiency of Fenticonazole for the Treatment of Vaginal Candidiasis. Srpski Arhiv Za Celokupno Lekarstvo 2012;140(7-8):469-474. - PubMed
References to ongoing studies
EUCTR2019‐000925‐27‐SK {published data only}
-
- A phase IIb/III, parallel-arm, randomized, active-controlled, double-blind, double-dummy, multicenter, non-inferiority study in patients with recurrent vulvovaginal candidosis to compare the clinical efficacy, safety and tolerability of topically administered ProF-001 (Candiplus®) to oral fluconazole. ICTRP. [URL: https://trialsearch.who.int/?TrialID=EUCTR2019-000925-27-SK]
-
- A Phase IIb/III Study of Prof-001 for the Treatment of Patients With Recurrent Vulvovaginal Candidiasis (RVVC). clinicaltrials.gov. [URL: https://clinicaltrials.gov/ct2/show/NCT04734405]
NCT04029116 {published data only}
-
- A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Oral Ibrexafungerp (SCY-078) Compared to Placebo in Subjects With Recurrent Vulvovaginal Candidiasis (VVC). ClinicalTrials.gov. [https://clinicaltrials.gov/ct2/show/NCT04029116]
NCT04208555 {published data only}
-
- A Randomized Comparison of Boric Acid Versus Terconazole in Treatment of Recurrent Vulvovaginal Candidiasis. ClinicalTrials.gov. [URL: https://clinicaltrials.gov/ct2/show/NCT04208555]
NCT04292704 {published data only}
-
- NCT04292704. clinicaltrials.gov. [URL: https://clinicaltrials.gov/ct2/show/record/NCT04292704]
Additional references
Beigi 2004
-
- Beigi RH, Meyn LA, Moore DM, Krohn MA, Hillier SL.Vaginal yeast colonization in nonpregnant women: a longitudinal study. Obstetrics & Gynecology 2004;104(5 Pt 1):926-930. - PubMed
CDC 2015
-
- Vulvovaginal candidiasis. Sexually Transmitted Diseases Treatment Guidelines 2015. [https://www.cdc.gov/std/tg2015/candidiasis.htm] - PMC - PubMed
Chapple 2000
-
- Chapple A, Hassell K, Nicholson M, Cantrill J."You don't really feel you can function normally": women's perceptions and personal management of vaginal thrush". Journal of Reproductive and Infant Psychology 2000;18(4):309-319.
Chen 2007
-
- Chen S, Sorrell T.Antifungal agents. Medical Journal of Australia 2007;187(7):404-409. - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JPT, Altman DG, editor(s).Chapter 9: Analysing data and undertaking metaanalyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Denning 2018
Ehrstrom 2007
-
- Ehrstrom S, Kornfeld D, Rylander E.Perceived stress in women with recurrent vulvovaginal candidiasis. Journal of Psychosomatic Obstetrics and Gynecology 2007;28(3):169-176. - PubMed
Falagas 2006
-
- Falagas M, Betsi GI, Athanasiou S.Probiotics for prevention of recurrent vulvovaginal candidiasis: A review. Journal of Antimicrobial Chemotherapy 2006;58:266-72. - PubMed
Fidel 1998
-
- Fidel PL, Sobel JD.Protective immunity in experimental Candida vaginitis. Research in Immunology 1998;149(4-5):361-73. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT.Hamilton (ON): McMaster University (developed by Evidence Prime), 2014. Available at gradepro.org.
Guaschino 2001
-
- Guaschino S, De Seta F, Sartore A, Ricci G, De Santo D, Piccoli M, Alberico S.Efficacy of maintenance therapy with topical boric acid in comparison with oral itraconazole in the treatment of recurrent vulvovaginal candidiasis. American Journal of Obstetrics and Gynecology 2001;184(4):598-602. [ISSN 002-9378] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. In: The Cochrane Collaboration. The Cochrane Collaboration, 2011. [Available from www.cochrane-handbook.org.]
Lopez 2013
-
- Lopez JEM.Candidiasis (vulvovaginal). BMJ Clin Evid 2013;3:0815.
Merkus 1990
-
- Merkus JM, Van Heusden AM.Chronic vulvovaginal candidosis: the role of oral treatment. British Journal of Clinical Practice 1990;71:s81-84. - PubMed
Miller 2000
-
- Miller L, Patton DL, Meier A, Thwin S, Hooton TM, Eschenbach DA.Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstetrics & Gynecology 2000;96(3):431-9. - PubMed
Moraes 2000
-
- Moraes PS, Lima Goiaba S, Taketomi EA.Candida albicans allergen immunotherapy in recurrent vaginal candidiasis. Journal of Investigational Allergology & Clinical Immunology 2000;10(5):305-9. - PubMed
Nurbhai 2007
-
- Nurbhai M, Grimshaw J, Watson M, Bond CM, Mollison JA, Ludbrook A.Oral versus intra-vaginal imidazole and triazole anti-fungal treatment of uncomplicated vulvovaginal candidiasis (thrush). Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD002845. [DOI: 10.1002/14651858.CD002845.pub2] - DOI - PubMed
Nyirjesy 2001
-
- Nyirjesy P.Chronic vulvovaginal candidiasis. American Family Physician 2001;63(4):697-702. - PubMed
Nyirjesy 2008
-
- Nyirjesy P.Vulvovaginal candidiasis and bacterial vaginosis. Infectious Disease Clinics of North America 2008;22(4):637-52. - PubMed
Odds 2003
-
- Odds FC, Brown AJP, Gow NAR.Antifungal agents: mechanisms of action. Trends in Microbiology 2003;11(6):272-9. - PubMed
Pappas 2015
Patel 2004
-
- Patel DA, Gillespie B, Sobel JD, Leaman D, Nyirjesy P, Weitz MV, Foxman B.Risk factors for recurrent vulvovaginal candidiasis in women receiving maintenance antifungal therapy: results of a prospective cohort study. American Journal of Obstetrics and Gynecology 2004;190(3):644-53. - PubMed
Peters 2014
Pirotta 2003
-
- Pirotta MV, Gunn JM, Chondros P."Not thrush again!" Women's experience of post-antibiotic vulvovaginitis. Medical Journal of Australia 2003;179(1):43-46. - PubMed
Pirotta 2004
Pontes 2014
-
- Pontes AC, Amaral RLG, Giraldo PC, Beghini J, Giraldo HPD, Cordeiro ES.A systematic review of the effect of daily panty liner use on the vulvovaginal environment. International Journal of Gynecology and Obstetrics 2014;127:1-5. - PubMed
Rathod 2014
Ray 2011
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5).Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosa 2013
-
- Rosa MI, Silva BR, Pires PS, Silva FR, Silva NC, Silva FR, et al.Weekly fluconazole therapy for recurrent vulvovaginal candidiasis: a systematic review and meta-analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology 2013;167:132-136. - PubMed
Shahid 2009
-
- Shahid Z, Sobel JD.Reduced fluconazole susceptibility of Candida albicans isolates in women with recurrent vulvovaginal candidiasis: effects of long-term fluconazole therapy. Diagnostic Microbiology and Infectious Disease 2009;64(3):354-56. - PubMed
Sobel 1995
-
- Sobel JD, Brooker D, Stein GE, Thomason JL, Wermeling DP, Bradley B, Weinstein L.Single oral dose fluconazole compared with conventional clotrimazole topical therapy of candida-vaginitis. American Journal of Obstetrics and Gynecology 1995;172(4):1263-68. - PubMed
Sobel 2004b
-
- Sobel JD, Wiesenfeld HC, Martens M, Danna P, Hooton TM, Rompalo A, et al.Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. New England Journal of Medicine 2004;351(9):876-83.. - PubMed
Sobel 2006
-
- Sobel JD.Management of recurrent vulvovaginal candidiasis: unresolved issues. Current Infectious Disease Reports 2006;8(6):481-86. - PubMed
Sobel 2007
-
- Sobel JD.Vulvovaginal candidosis. Lancet 2007;369(9577):1961-71. - PubMed
Sobel 2014
Sobel 2016
-
- Sobel JD.Recurrent vulvovaginal candidiasis. American Journal of Obstetrics & Gynecology 2016;214(1):15-21. - PubMed
Stein 1993
Therapeutic Guidelines 2015
-
- Therapeutic Guidelines.eTG: Antibiotic. updated June 2019 edition. Melbourne, Victoria: Therapeutic Guidelines Limited, 2015.
Van Kessel 2003
-
- Van Kessel K, Assefi N, Marrazzo J, Eckert L.Common complementary and alternative therapies for yeast vaginitis and bacterial vaginosis: A systematic review. Obstetrical & Gynecological Survey 2003;58(5):351-58. - PubMed
Watson 2002
-
- Watson MC, Grimshaw JM, Bond CM, Mollison J, Ludbrook A.Oral versus intra-vaginal imidazole and triazole anti-fungal agents for the treatment of uncomplicated vulvovaginal candidiasis (thrush): a systematic review. British Journal of Obstetrics and Gynaecology 2002;109:85-95. - PubMed
Watson 2007
-
- Watson C, Calabretto H.Comprehensive review of conventional and non-conventional methods of management of recurrent vulvovaginal candidiasis. Australian and New Zealand Journal of Obstetrics and Gynaecology 2007;47:262-272. - PubMed
Watson 2011
-
- Watson C, Pirotta M.Recurrent vulvovaginal candidiasis - current management. Australian Family Physician March 2011;40(3):149-151. - PubMed
Wildfeuer 1997
-
- Wildfeuer A, Laufen H, Schmalreck AF, Yeates RA, Zimmermann T.Fluconazole: comparison of pharmacokinetics, therapy and in vitro susceptibility. Mycoses 1997;40(7-8):259-65. - PubMed
Xie 2017
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

