Systemic therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer
- PMID: 35005781
- PMCID: PMC8743877
- DOI: 10.1002/14651858.CD013167.pub2
Systemic therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer
Abstract
Background: Adjuvant aromatase inhibitors (AI) improve survival compared to tamoxifen in postmenopausal women with hormone receptor-positive stage I to III breast cancer. In approximately half of these women, AI are associated with aromatase inhibitor-induced musculoskeletal symptoms (AIMSS), often described as symmetrical pain and soreness in the joints, musculoskeletal pain and joint stiffness. AIMSS may have significant and prolonged impact on women's quality of life. AIMSS reduces adherence to AI therapy in up to a half of women, potentially compromising breast cancer outcomes. Differing systemic therapies have been investigated for the prevention and treatment of AIMSS, but the effectiveness of these therapies remains unclear.
Objectives: To assess the effects of systemic therapies on the prevention or management of AIMSS in women with stage I to III hormone receptor-positive breast cancer.
Search methods: We searched CENTRAL, MEDLINE, Embase, WHO International Clinical Trials Registry Platform (ICTRP) and Clinicaltrials.gov registries to September 2020 and the Cochrane Breast Cancer Group (CBCG) Specialised Register to March 2021. SELECTION CRITERIA: We included all randomised controlled trials that compared systemic therapies to a comparator arm. Systemic therapy interventions included all pharmacological therapies, dietary supplements, and complementary and alternative medicines (CAM). All comparator arms were allowed including placebo or standard of care (or both) with analgesia alone. Published and non-peer-reviewed studies were eligible.
Data collection and analysis: Two review authors independently screened studies, extracted data, and assessed risk of bias and certainty of the evidence using the GRADE approach. Outcomes assessed were pain, stiffness, grip strength, safety data, discontinuation of AI, health-related quality of life (HRQoL), breast cancer-specific quality of life (BCS-QoL), incidence of AIMSS, breast cancer-specific survival (BCSS) and overall survival (OS). For continuous outcomes, we used vote-counting by reporting how many studies reported a clinically significant benefit within the confidence intervals (CI) of the mean difference (MD) between treatment arms, as determined by the minimal clinically importance difference (MCID) for that outcome scale. For dichotomous outcomes, we reported outcomes as a risk ratio (RR) with 95% CI.
Main results: We included 17 studies with 2034 randomised participants. Four studies assessed systemic therapies for the prevention of AIMSS and 13 studies investigated treatment of AIMSS. Due to the variation in systemic therapy studies, including pharmacological, and CAM, or unavailable data, meta-analysis was limited, and only two trials were combined for meta-analysis. The certainty of evidence for all outcomes was either low or very low certainty. Prevention studies The evidence is very uncertain about the effect of systemic therapies on pain (from baseline to the end of the intervention; 2 studies, 183 women). The two studies, investigating vitamin D and omega-3 fatty acids, showed a treatment effect with 95% CIs that did not include an MCID for pain. Systemic therapies may have little to no effect on grip strength (RR 1.08, 95% CI 0.37 to 3.17; 1 study, 137 women) or on women continuing to take their AI (RR 0.16, 95% 0.01 to 2.99; 1 study, 147 women). The evidence suggests little to no effect on HRQoL and BCS-QoL from baseline to the end of intervention (the same single study; 44 women, both quality of life outcomes showed a treatment effect with 95% CIs that did include an MCID). The evidence is very uncertain for outcomes assessing incidence of AIMSS (RR 0.82, 95% CI 0.63 to 1.06; 2 studies, 240 women) and the safety of systemic therapies (4 studies, 344 women; very low-certainty evidence). One study had a US Food and Drug Administration alert issued for the intervention (cyclo-oxygenase-2 inhibitor) during the study, but there were no serious adverse events in this or any study. There were no data on stiffness, BCSS or OS. Treatment studies The evidence is very uncertain about the effect of systemic therapies on pain from baseline to the end of intervention in the treatment of AIMSS (10 studies, 1099 women). Four studies showed an MCID in pain scores which fell within the 95% CI of the measured effect (vitamin D, bionic tiger bone, Yi Shen Jian Gu granules, calcitonin). Six studies showed a treatment effect with 95% CI that did not include an MCID (vitamin D, testosterone, omega-3 fatty acids, duloxetine, emu oil, cat's claw). The evidence was very uncertain for the outcomes of change in stiffness (4 studies, 295 women), HRQoL (3 studies, 208 women) and BCS-QoL (2 studies, 147 women) from baseline to the end of intervention. The evidence suggests systemic therapies may have little to no effect on grip strength (1 study, 107 women). The evidence is very uncertain about the safety of systemic therapies (10 studies, 1250 women). There were no grade four/five adverse events reported in any of the studies. The study of duloxetine reported more all-grade adverse events in this treatment group than comparator group. There were no data on the incidence of AIMSS, the number of women continuing to take AI, BCCS or OS from the treatment studies.
Authors' conclusions: AIMSS are chronic and complex symptoms with a significant impact on women with early breast cancer taking AI. To date, evidence for safe and effective systemic therapies for prevention or treatment of AIMSS has been minimal. Although this review identified 17 studies with 2034 randomised participants, the review was challenging due to the heterogeneous systemic therapy interventions and study methodologies, and the unavailability of certain trial data. Meta-analysis was thus limited and findings of the review were inconclusive. Further research is recommended into systemic therapy for AIMSS, including high-quality adequately powered RCT, comprehensive descriptions of the intervention/placebo, and robust definitions of the condition and the outcomes being studied.
Trial registration: ClinicalTrials.gov NCT01809171 NCT03384095 NCT02831582.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
KER: travel/accommodation/meeting expenses by Roche, Amgen, Pfizer; advisory board Amgen; conference registration by Novartis.
IA: none.
KR: none.
SC: none.
MC: none.
NW: consultancy fees by Roche and Novartis; grant for department trials from Medivation; expert panel review for Roche; stock in CSL, travel/accommodation/meeting expenses by Roche and Novartis.
Figures
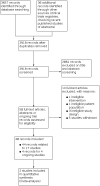














Update of
- doi: 10.1002/14651858.CD013167
Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Interventions for the treatment of brain radionecrosis after radiotherapy or radiosurgery.Cochrane Database Syst Rev. 2018 Jul 9;7(7):CD011492. doi: 10.1002/14651858.CD011492.pub2. Cochrane Database Syst Rev. 2018. PMID: 29987845 Free PMC article.
-
Acupuncture for treating overactive bladder in adults.Cochrane Database Syst Rev. 2022 Sep 23;9(9):CD013519. doi: 10.1002/14651858.CD013519.pub2. Cochrane Database Syst Rev. 2022. PMID: 36148895 Free PMC article.
-
Physical activity for treatment of irritable bowel syndrome.Cochrane Database Syst Rev. 2022 Jun 29;6(6):CD011497. doi: 10.1002/14651858.CD011497.pub2. Cochrane Database Syst Rev. 2022. PMID: 35766861 Free PMC article.
Cited by
-
Insights and implications of sexual dimorphism in osteoporosis.Bone Res. 2024 Feb 18;12(1):8. doi: 10.1038/s41413-023-00306-4. Bone Res. 2024. PMID: 38368422 Free PMC article. Review.
-
Dietary Supplement Use in Women Diagnosed with Breast Cancer.J Nutr. 2023 Jan;153(1):301-311. doi: 10.1016/j.tjnut.2022.12.007. Epub 2022 Dec 26. J Nutr. 2023. PMID: 36913466 Free PMC article.
-
What are the effects of systemic therapies for the prevention and treatment of aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer? - A Cochrane Review summary with commentary.J Musculoskelet Neuronal Interact. 2022 Sep 1;22(3):301-304. J Musculoskelet Neuronal Interact. 2022. PMID: 36046985 Free PMC article. No abstract available.
-
Aromatase Inhibitor Musculoskeletal Syndrome and Bone Loss: a Review of the Current Literature.Curr Oncol Rep. 2023 Jul;25(7):825-831. doi: 10.1007/s11912-023-01413-5. Epub 2023 Apr 13. Curr Oncol Rep. 2023. PMID: 37052869 Review.
-
Acupoint Thread Embedding Combined With Wenshen Bugu Decoction for the Treatment of Aromatase Inhibitor-Associated Musculoskeletal Symptom Among Postmenopausal Breast Cancer Patients: Study Protocol of a Randomized Controlled Trial.Integr Cancer Ther. 2023 Jan-Dec;22:15347354231188679. doi: 10.1177/15347354231188679. Integr Cancer Ther. 2023. PMID: 37565358 Free PMC article.
References
References to studies included in this review
Birrell 2009 {published data only (unpublished sought but not used)}
-
- Birrell S, Tilley W. Testosterone undecanoate treatment reduces joint morbidities induced by anastrozole therapy in postmenopausal women with breast cancer: results of a double-blind, randomized phase II trial. Cancer Research 2009;69(24 Suppl):Abstract no. 804.
-
- Birrell SN. Reduction of side effects from aromatase inhibitors used for treating breast cancer. United States Patent Application Publication (date published 17 May 2012):US 2012/0122824 A1.
-
- NCT00497458. Androgen therapy for breast cancer patients with aromatase inhibitor induced side effects. clinicaltrials.gov/show/NCT00497458 (first received 6 July 2007).
Cathcart‐Rake 2020 {published data only}
-
- Cathcart-Rake E, Novotny P, Leon-Ferre R, Le-Rademacher J, Storrick EM, Adjei AA, et al. A randomized, double-blind, placebo controlled trial of testosterone for treatment of postmenopausal women with aromatase inhibitor-induced arthralgias: Alliance study A221102. Supportive Care in Cancer 2020;29(1):387-96. [DOI: 10.1007/s00520-020-05473-2.] - DOI - PMC - PubMed
-
- Leon-Ferre RA, Le-Rademacher J, Terstriep S, Glaser R, Novotni P, Giuliano A, et al. A randomized, double-blind, placebo-controlled trial of testosterone (T) for aromatase inhibitor-induced arthralgias (AIA) in postmenopausal women: Alliance A221102. Cancer Research 2019;79(4 Suppl):P4-16-01. - PMC - PubMed
-
- NCT01573442. Testosterone in treating postmenopausal patients with arthralgia caused by adjuvant aromatase inhibitor treatment. clinicaltrials.gov/show/NCT01573442 (first received 9 April 2012).
Chan 2017 {published and unpublished data}
-
- ACTRN12611000019909. Emu oil for joints pain in postmenopausal women with early breast cancer. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336281 (first received 1 December 2010).
-
- Chan A, De Boer R, Gan A, Willsher P, Martin R, Zissiadis Y, et al. Randomized phase II placebo-controlled study to evaluate the efficacy of topical pure emu oil for joint pain related to adjuvant aromatase inhibitor use in postmenopausal women with early breast cancer: JUST (Joints Under Study). Supportive Care in Cancer 2017;25(12):3785-91. - PubMed
Henry 2018 {published data only (unpublished sought but not used)}
-
- Henry NL, Unger JM, Schott AF, Fehrenbacher L, Flynn PJ, Prow DM, et al. Randomized, multicenter, placebo-controlled clinical trial of duloxetine versus placebo for aromatase inhibitor-associated arthralgias in early-stage breast cancer: SWOG S1202. Journal of Clinical Oncology 2018;36(4):326‐32. - PMC - PubMed
-
- NCT01598298. S1202: duloxetine hydrochloride for muscle/joint pain in early-stage breast cancer receiving hormone therapy. clinicaltrials.gov/show/NCT01598298 (first received 15 May 2012).
Hershman 2015a {published data only (unpublished sought but not used)}
-
- Hershman DL, Unger JM, Crew KD, Dakhil SR, Awad D, Greenlee H, et al. Omega-3 fatty acids for aromatase inhibitor-induced musculoskeletal symptoms in women with early-stage breast cancer (SWOG S0927). Journal of Clinical Oncology 2014;32(15 Suppl):Abstract no. 9532.
-
- Hershman DL, Unger JM, Crew KD, Moinpour CM, Minasian LM, Hansen L, et al. SWOG S0927: a randomized double blind placebo-controlled trial of omega-3-fatty acid for the control of aromatase inhibitor (AI)-induced musculoskeletal pain in women with early stage breast cancer. Cancer Research 2011;71(24 Suppl):Abstract no. OT2-07-02.
-
- NCT01385137. S0927: a randomized placebo-controlled trial of omega-3-fatty acid for the control of aromatase inhibitor-induced musculoskeletal pain and stiffness in women with early stage breast cancer, phase III. clinicaltrials.gov/ct2/show/NCT01385137 (first received 29 June 2011).
Khan 2017 {published and unpublished data}
-
- Khan QJ, Kimler BF, Reddy PS, Sharma P, Klemp JR, Fabian CJ. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms and fatigue in women with breast cancer starting adjuvant letrozole: the VITAL trial. Journal of Clinical Oncology 2012;30(15 Suppl):Abstract no. 9000. - PubMed
-
- Khan QJ, Kimler BF, Reddy PS, Sharma P, Klemp JR, Nydegger JL, et al. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms in women with breast cancer receiving adjuvant letrozole. The VITAL trial. Breast Cancer Research and Treatment 2017;166(2):491-500. - PubMed
-
- NCT00867217. Vitamin D3 for aromatase inhibitor induced arthralgias. clinicaltrials.gov/show/NCT00867217 (first received 23 March 2009).
Li 2017 {published data only (unpublished sought but not used)}
Liu 2014 {published data only (unpublished sought but not used)}
-
- Liu P, Yang DQ, Xie F, Zhou B, Liu M. Effect of calcitonin on anastrozole-induced bone pain during aromatase inhibitor therapy for breast cancer. Genetics and Molecular Research 2014;13(3):5285-91. - PubMed
Lustberg 2018 {published data only (unpublished sought but not used)}
-
- Lustberg MB, Orchard T, Pan X, Reinbolt R, Logan A, Lester J, et al. Prevention of aromatase Inhibitor (AI)-induced joint symptoms with omega-3 fatty acid supplementation: a randomized placebo-controlled pilot study. Cancer Research 2015;75(9 Suppl):Abstract no. P1-09-03.
-
- NCT01478477. Omega-3 fatty acids in preventing joint symptoms in patients with stage I-III breast cancer receiving anastrozole, exemestane, or letrozole. clinicaltrials.gov/show/NCT01478477 (first received 23 November 2011).
-
- Orchard TS, Pan X, Lester J, Logan A, Shapiro CL, Jackson RD, et al. Relationship of dietary and red blood cell polyunsaturated fatty acids to inflammatory markers in breast cancer survivors taking aromatase inhibitors. Cancer Research 2015;75(9 Suppl):Abstract no. 14-P1-09-30.
Massimino 2011 {published data only (unpublished sought but not used)}
-
- Massimino K, Glissmeyer M, Wagie T, Karamlou K, Look RM, Sorenson L, et al. Use of Blue Citrus, a Chinese herbal remedy, to reduce side effects of aromatase inhibitors. Journal of Clinical Oncology 2011;29(27 Suppl):Abstract no. 170.
-
- NCT00702858. Trial of Blue Citrus compared to placebo in patients receiving aromatase inhibitor therapy for estrogen receptor positive post-menopausal breast cancer. clinicaltrials.gov/show/NCT00702858 (first received 20 June 2008).
Niravath 2019 {published and unpublished data}
-
- NCT01988090. High dose vitamin D vs standard dose vitamin D study. clinicaltrials.gov/ct2/show/NCT01988090 (first received 20 November 2013).
-
- Niravath P, Hilsenbeck SG, Wang T, Jiralerspong S, Nangia J, Pavlick A, et al. Randomized controlled trial of high-dose versus standard-dose vitamin D3 for prevention of aromatase inhibitor-induced arthralgia. Breast Cancer Research and Treatment 2019;177(2):427‐35. - PubMed
-
- Niravath PA, Hilsenbeck S, Wang T, Rimawi M. A randomized, controlled trial of high dose vs. standard dose vitamin D for aromatase inhibitor-induced arthralgia in breast cancer survivors. Cancer Research 2014;74(19 Suppl):Abstract no. CT319. - PubMed
Peng 2018 {published data only (unpublished sought but not used)}
-
- ISRCTN06129599. Traditional Chinese medicine for the management of aromatase inhibitor-associated musculoskeletal symptoms. isrctn.com/ISRCTN06129599 (first received 24 July 2013).
-
- Peng N, Yu M, Yang G, Fu Q, Xu Y, Yu J, et al. Effects of the Chinese medicine Yi Shen Jian Gu granules on aromatase inhibitor-associated musculoskeletal symptoms: a randomized, controlled clinical trial. Breast 2018;37:18-27. - PubMed
Rastelli 2011 {published data only (unpublished sought but not used)}
-
- NCT00263185. High dose vit D musculoskeletal symptoms & bone density in anastrozole-treated breast cancer with marginal vit D status. clinicaltrials.gov/show/NCT00263185 (first received 7 December 2005).
-
- Rastelli A, Taylor M, Villareal R, Jamalabadi-Majidi S, Gao F, Ellis M. A double-blind, randomized, placebo-controlled trial of high dose vitamin D therapy on musculoskeletal pain and bone mineral density in anastrozole-treated breast cancer patients with marginal vitamin D status. Cancer Research 2010;69(24 Suppl):Abstract no. 803.
-
- Rastelli AL, Taylor ME, Gao F, Armamento-Villareal R, Jamalabadi-Majidi S, Napoli N, et al. Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): a phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Research and Treatment 2011;129(1):107-16. - PubMed
Rosati 2011 {published data only (unpublished sought but not used)}
-
- Rosati MS, Di Seri M, Baciarello G, Lo Russo V, Grassi P, Marchetti L, et al. Etoricoxib and anastrozole in adjuvant early breast cancer: ETAN trial (phase III). Journal of Clinical Oncology 2011;29(15 Suppl):Abstract no. 533.
Shapiro 2016 {published data only}
-
- NCT01509079. Vitamin D3 effects on musculoskeletal symptoms with use of aromatase inhibitors. clinicaltrials.gov/ct2/show/NCT01509079 (first received 12 January 2012).
-
- Shapiro A, Adlis S, Robien K, Kirstein M, Liang S, Richter S, et al. Erratum: randomized, blinded trial of vitamin D3 for treating aromatase inhibitor-associated musculoskeletal symptoms (AIMSS). Breast Cancer Research and Treatment 2016;157(2):403. - PubMed
-
- Shapiro AC, Adlis SA, Liang S, Robien K, Kirstein MN, Anderson E, et al. A randomized trial of vitamin D3 in aromatase inhibitor-associated musculoskeletal symptoms. Journal of Clinical Oncology 2015;33(15 Supp):Abstract no. 9608.
-
- Shapiro AC, Kirstein MN, Robien K, Swenson KK, Nissen MJ, Menk JS, et al. Vitamin D3 supplementation, musculoskeletal (MS) symptoms and aromatase inhibitor (AI) pharmacokinetics from the vitamin D3 AI study. Cancer Research 2013;73(24 Suppl):Abstract no. P5-09-18.
Shenouda 2019 {published data only (unpublished sought but not used)}
-
- Shenouda M, Tria Tirona MR. Effect of tart cherry on aromatase inhibitor-induced arthralgia (AIA) in nonmetastatic hormone-positive breast cancer patients: a randomized double-blind placebo-controlled trial. Journal of Clinical Oncology 2019;37(15 Suppl):Abstract no. 525. - PubMed
Sordi 2019 {published and unpublished data}
-
- Sordi R, Castro SN, Lera AT, Irene MN, Farinazzo MM, Sette C, et al. Randomized, double-blind, placebo-controlled phase ii clinical trial on the use of Uncaria tomentosa (Cat's claw) for aromatase inhibitor-induced arthralgia: a pilot study. Journal of Natural Remedies 2019;19(1):24-31.
References to studies excluded from this review
ACTRN12611000891921 {published data only}
-
- ACTRN12611000891921. A trial to determine the effect of glucosamine versus placebo on aromatase inhibitor induced arthralgia (joint pain) in postmenopausal women with early breast cancer who are on letrozole. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12611000891921 (first received 9 August 2011).
Altundag 2019 {published data only}
-
- Altundag K. Combined use of vitamin D and omega-3 fatty acid in breast cancer patients might be more beneficial for reducing aromatase inhibitors-associated arthralgia. Journal of BUON 2019;24(2):862. - PubMed
Barbor 2018 {published data only}
-
- Barbor M. Omega-3 fatty acids significantly reduced pain in obese patients with breast cancer. Oncology Nurse-APN/PA 2018;11(5):1-6.
Becze 2017 {published data only}
-
- Becze E. Antidepressant may relieve joint pain from aromatase inhibitors. ONS Voice 2017;32(3):11-11.
KCT0003698 {published data only}
-
- KCT0003698. Effect of moxibustion therapy for aromatase inhibitor-induced arthralgia of postmenopausal breast cancer patients. apps.who.int/trialsearch/Trial2.aspx?TrialID=KCT0003698 (date of registration 1 April 2019).
Kim 2012 {published data only}
-
- Kim HA, Choi HJ, Lim MJ, Park W, Lee J, Choi SJ, et al. Nonsteroidal antiinflammatory drugs (NSAID) versus NSAID with hydroxychloroquine in treatment of chemotherapy-related arthropathy: open-label multicenter pilot study. Journal of Rheumatology 2012;39(9):1902-3. - PubMed
NCT01809171 {published data only}
-
- NCT01809171. Placebo-controlled trial with vitamin D to prevent worsening/relieve aromatase inhibitor-induced musculoskeletal symptoms in breast cancer patients. clinicaltrials.gov/ct2/show/NCT01809171 (first received 12 March 2013).
NCT03384095 {published data only}
-
- NCT03384095. Trial of oral hyaluronic acid for the prevention of aromatase inhibitor-associated arthralgias. clinicaltrials.gov/ct2/show/NCT03384095 (first received 27 December 2017).
UMIN000004416 {published data only}
-
- UMIN000004416. Japanese study for usefulness of vitamin E in reducing aromatase inhibitor-associated joint symptoms. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_his_list.cgi?recptno=R000005255 (date uploaded 19 October 2010). [TRIAL REGISTRY: UMIN000004416]
References to ongoing studies
NCT02831582 {published data only}
-
- NCT02831582. Omega-3 supplementation in prevention of aromatase inhibitor-induced toxicity in patients with stage I–III breast cancer. clinicaltrials.gov/show/NCT02831582 (first received 13 July 2016).
NCT03865992 {published data only}
-
- NCT03865992. Curcumin in reducing joint pain in breast cancer survivors with aromatase inhibitor-induced joint disease. clinicaltrials.gov/ct2/show/NCT03865992 (first received 7 March 2019).
NCT04205786 {published data only}
-
- NCT04205786. Vitamin B12 for aromatase inhibitors associated musculoskeletal symptoms in breast cancer. clinicaltrials.gov/ct2/show/NCT04205786 (first received 19 December 2019).
UMIN000027481 {published data only}
-
- UMIN000027481. Randomized study of Sokei-Kakketsu-To for the reduction of aromatase inhibitor – associated joint symptoms in women with breast cancer. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000031481 (first received 25 May 2017).
Additional references
Aguilar 2002
-
- Aguilar JL, Rojas P, Marcelo A, Plaza A, Bauer R, Reininger E, et al. Anti-inflammatory activity of two different extracts of Uncaria tomentosa (Rubiaceae). Journal of Ethnopharmacology 2002;81(2):271-6. - PubMed
Ambrosone 2020
Aquino 1989
-
- Aquino R, De Simone F, Pizza C, Conti C, Stein ML. Plant metabolites. Structure and in vitro antiviral activity of quinovic acid glycosides from Uncaria tomentosa and Guettarda platypoda. Journal of Natural Products 1989;52(4):679-85. - PubMed
Arnold 2005
-
- Arnold LM, Rosen A, Pritchett YL, D'Souza DN, Goldstein DJ, Iyengar S, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain 2005;119(1-3):5-15. - PubMed
Basch 2010
Basch 2016
Basch 2017
Beckwee 2017
-
- Beckwee D, Leysen L, Meuwis K, Adriaenssens N. Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: a systematic review and meta-analysis. Supportive Care in Cancer 2017;25(5):1673-86. - PubMed
Bellingham 2010
-
- Bellingham GA, Peng PW. Duloxetine: a review of its pharmacology and use in chronic pain management. Regional Anaesthesia and Pain Medication 2010;35(3):294-303. - PubMed
Bhandari 2004
Bohannon 2019
Boon 2000
-
- Boon H, Stewart M, Kennard MA, Gray R, Sawka C, Brown JB, et al. Use of complementary/alternative medicine by breast cancer survivors in Ontario: prevalence and perceptions. Journal of Clinical Oncology 2000;18(13):2515-21. - PubMed
Boon 2007
Borrie 2017
-
- Borrie AE, Kim RB. Molecular basis of aromatase inhibitor associated arthralgia: known and potential candidate genes and associated biomarkers. Journal of Expert Opinion on Drug Metabolism & Toxicology 2017;13(2):149-56. - PubMed
Brandt 2019
-
- Brandt J, Scotté F, Jordan K. Patient-reported outcomes (PROs) as a routine measure for cancer inpatients: the final missing piece of the puzzle? Annals of Oncology 2019;30(2):167-9. - PubMed
Brier 2017
Briot 2010
-
- Briot K, Tubiana-Hulin M, Bastit L, Kloos I, Roux C. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (articular tolerance of letrozole) study. Breast Cancer Research and Treatment 2010;120(1):127-34. - PubMed
Burstein 2007
-
- Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast 2007;16(3):223-34. - PubMed
Burstein 2019
-
- Burstein HJ, Lacchetti C, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO Clinical Practice Guideline Focused Update. Journal of Oncology Practice 2019;15(2):106-7. - PubMed
Castel 2013
Castel 2015
Chen 2008
Chen 2017
-
- Chen L, Lin CC, Huang TW, Kuan YC, Huang YH, Chen HC, et al. Effect of acupuncture on aromatase inhibitor-induced arthralgia in patients with breast cancer: a meta-analysis of randomized controlled trials. Breast 2017;33:132-8. - PubMed
Chesnut 2008
-
- Chesnut CH, Azria M, Silverman S, Engelhardt M, Olson M, Mindeholm L. Salmon calcitonin: a review of current and future therapeutic indications. Osteoporosis International 2008;19(4):479-91. - PubMed
Chim 2013
Chirgwin 2016
-
- Chirgwin JH, Giobbie-Hurder A, Coates AS, Price KN, Ejlertsen B, Debled M, et al. Treatment adherence and Its Impact on disease-free survival in the Breast International Group 1-98 Trial of tamoxifen and letrozole, alone and in sequence. Journal of Clinical Oncology 2016;34(21):2452-9. - PMC - PubMed
Chlebowski 2011
Cleeland 2009
-
- Cleeland CS. The Brief Pain Inventory: user guide, 2009. www.mdanderson.org/documents/Departments-and-Divisions/Symptom-Research/... (accessed 18 December 2021).
Cleland 1988
-
- Cleland LG, French JK, Betts WH, Murphy GA, Elliott MJ. Clinical and biochemical effects of dietary fish oil supplements in rheumatoid arthritis. Journal of Rheumatology 1988;15(10):1471-5. - PubMed
CMSG 2021
-
- Cochrane Musculoskeletal Group. Proposed outcomes. musculoskeletal.cochrane.org/proposed-outcomes (accessed 16 October 2021).
Crew 2007
-
- Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. Journal of Clinical Oncology 2007;25(25):3877-83. - PubMed
Crew 2009
Cruciani 2012
-
- Cruciani RA, Zhang JJ, Manola J, Cella D, Ansari B, Fisch MJ. L-carnitine supplementation for the management of fatigue in patients with cancer: an Eastern Cooperative Oncology Group phase III, randomized, double-blind, placebo-controlled trial. Journal of Clinical Oncology 2012;30(31):3864-9. - PMC - PubMed
Cui 2004
-
- Cui Y, Shu XO, Gao Y, Wen W, Ruan ZX, Jin F, et al. Use of complementary and alternative medicine by Chinese women with breast cancer. Breast Cancer Research and Treatment 2004;85(3):263-70. - PubMed
Da Costa 2014
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021 Available from www.training.cochrane.org/handbook.
Delgado 2017
Deshpande 2011
Dworkin 2009
-
- Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146(3):238-44. - PubMed
EBCTCG 2015
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386(10001):1341-52. - PubMed
Ervin 2004
-
- Ervin RB, Wright JD, Wang C, Kennedy-Stephenson J. Dietary intake of fats and fatty acids for the United States population: 1999–2000. Advance Data 2004;348:1-6. - PubMed
Eton 2004
-
- Eton D, Cella D, Jost K, Yount S, Peterman A, Neuberg D, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. Journal of Clinical Epidemiology 2004;57(9):898-910. [DOI: ] - PubMed
Excel [Computer program]
-
- Microsoft Excel. Version 16.17. Redmond (WA): Microsoft Corporation, 2018.
Farrar 2010
-
- Farrar JT, Pritchett YL, Robinson M, Prakash A, Chappell A. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. Journal of Pain 2010;11(2):109-18. - PubMed
FDA 2018
-
- Food and Drug Administration. COX-2 selective (includes Bextra, Celebrex, and Vioxx) and non-selective non-steroidal anti-inflammatory drugs (NSAIDs). www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-provid... (accessed 18 December 2021).
Felson 2005
-
- Felson DT, Cummings SR. Aromatase inhibitors and the syndrome of arthralgias with estrogen deprivation. Arthritis and Rheumatism 2005;52(9):2594-8. - PubMed
Ferlay 2012
-
- Ferlay J, Soerjomataram I, Ervik M. GLOBOCAN, Cancer Incidence and Mortality Worldwide. 1.0 edition. Lyon, France: International Agency for Research on Cancer, 2012.
Francis 2015
Francis 2018
Gall 2018
Gallagher 1980
-
- Gallagher JC, Lawrence Riggs B, Deluca HF. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. Journal of Clinical Endocrinology and Metabolism 1980;51(6):1359-64. - PubMed
Goldberg 2007
-
- Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 2007;129(1-2):210-23. - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed prior to 10 September 2018. Hamilton (ON): GRADE Working Group, McMaster University, 2014. Available at gradepro.org.
Greenlee 2012
Greenlee 2013
Gupta 2020
-
- Gupta A, Henry NL, Loprinzi CL. Management of aromatase inhibitor-induced musculoskeletal symptoms. JCO Oncology Practice 2020;16(11):733-9. - PubMed
Hack 2020
Hadji 2014
-
- Hadji P, Jackisch C, Bolten W, Blettner M, Hindenburg HJ, Klein P, et al. COMPliance and Arthralgia in Clinical Therapy: the COMPACT trial, assessing the incidence of arthralgia, and compliance within the first year of adjuvant anastrozole therapy. Annals of Oncology 2014;25(2):372-7. - PubMed
Henry 2008
Henry 2011
-
- Henry NL, Banerjee M, Wicha M, Poznak C, Smerage JB, Schott AF, et al. Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer 2011;117(24):5469-75. - PubMed
Henry 2012
Henry 2015
-
- Henry NL, Griggs JJ. The power of the placebo in symptom management. Journal of Clinical Oncology 2015;33(17):1870-2. - PubMed
Henry 2019
Hershman 2011
Hershman 2015a
Hershman 2015b
-
- Hershman DL, Loprinzi C, Schneider BP. Symptoms: aromatase inhibitor induced arthralgias. Advances in Experimental Medicine and Biology 2015;862:89-100. - PubMed
Hertz 2017
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2011b
-
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Higgins 2011c
-
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
-
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021), Cochrane, 2021. Available from training.cochrane.org/handbook.
Holick 2007
-
- Holick MF. Vitamin D deficiency. New England Journal of Medicine 2007;357(3):266-81. - PubMed
Howlader 2019
-
- Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M. Median age of cancer patients at diagnosis (2012–2016). SEER Cancer Statistics Review (CSR) 1975–2016 2019:seer.cancer.gov/archive/csr/1975_2016/. Table 1.11.
Kadakia 2016
Kamper 2009
Kanematsu 2011
Kelley 2018
Kim 2018
-
- Kim TH, Kang JW, Lee TH. Therapeutic options for aromatase inhibitor-associated arthralgia in breast cancer survivors: a systematic review of systematic reviews, evidence mapping, and network meta-analysis. Maturitas 2018;118:29-37. - PubMed
Kremser 2008
-
- Kremser T, Evans A, Moore A, Luxford K, Begbie S, Bensoussan A, et al. Use of complementary therapies by Australian women with breast cancer. Breast 2008;17(4):387-94. - PubMed
Kronenberg 2006
Kubo 2012
-
- Kubo M, Onishi H, Kuroki S, Okido M, Shimada K, Yokohata K, et al. Short-term and low-dose prednisolone administration reduces aromatase inhibitor-induced arthralgia in patients with breast cancer. Anticancer Research 2012;32(6):2331-6. - PubMed
Kwan 2017
Laroche 2017
Li 2013
Lilly 2020
-
- Lilly Pharmaceuticals USA. Highlights of prescribing information: CYMBALTA (duloxetine delayed-release capsules), for oral use. pi.lilly.com/us/cymbalta-pi.pdf (accessed 18 December 2021).
Lintermans 2013
-
- Lintermans A, Laenen A, Calster B, Hoydonck M, Pans S, Verhaeghe J, et al. Prospective study to assess fluid accumulation and tenosynovial changes in the aromatase inhibitor-induced musculoskeletal syndrome: 2-year follow-up data. Annals of Oncology 2013;24(2):350-5. - PubMed
Lintermans 2014
-
- Lintermans A, Asten K, Wildiers H, Laenen A, Paridaens R, Weltens C, et al. A prospective assessment of musculoskeletal toxicity and loss of grip strength in breast cancer patients receiving adjuvant aromatase inhibitors and tamoxifen, and relation with BMI. Breast Cancer Research and Treatment 2014;146(1):109-16. - PubMed
Lintermans 2016
-
- Lintermans A, Asten K, Jongen L, Brussel T, Laenen A, Verhaeghe J, et al. Genetic variant in the osteoprotegerin gene is associated with aromatase inhibitor-related musculoskeletal toxicity in breast cancer patients. European Journal of Cancer 2016;56:31-6. - PubMed
Lopez 2015
-
- Lopez C, Charles C, Rouby P, Boinon D, Laurent S, Rey A, et al. Relations between arthralgia and fear of recurrence: results of a cross-sectional study of breast cancer patients treated with adjuvant aromatase inhibitors therapy. Supportive Care in Cancer 2015;23(12):3581-8. - PubMed
Loprinzi 2009
Lustberg 2015
-
- Lustberg MB, Orchard T, Pan X, Reinbolt R, Logan A, Lester J, et al. Prevention of aromatase Inhibitor (AI)-induced joint symptoms with omega-3 fatty acid supplementation: a randomized placebo-controlled pilot study. Cancer Research 2015;75(9 Suppl):Abstract P1-09-03.
Lustberg 2018
Mao 2009
Mao 2011
Maredupaka 2020
Markham 2020
-
- Markham MJ, Wachter K, Agarwal N, Bertagnolli MM, Chang SM, Dale W, et al. Clinical cancer advances 2020: annual report on progress against cancer from the American Society of Clinical Oncology. Journal of Clinical Oncology 2020;38(10):1081. - PubMed
McKenzie 2021
-
- McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Mease 2011
-
- Mease PJ, Spaeth M, Clauw DJ, Arnold LM, Bradley LA, Russell IJ, et al. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care & Research 2011;63(6):821-6. - PubMed
Moy 2006
-
- Moy B, Tu D, Pater JL, Ingle JN, Shepherd LE, Whelan TJ, et al. Clinical outcomes of ethnic minority women in MA.17: a trial of letrozole after 5 years of tamoxifen in postmenopausal women with early stage breast cancer. Annals of Oncology 2006;17(11):1637-43. - PubMed
Nadji 2005
-
- Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. American Journal of Clinical Pathology 2005;123(1):21-7. - PubMed
Nahm 2018
-
- Nahm N, Mee S, Marx G. Efficacy of management strategies for aromatase inhibitor-induced arthralgia in breast cancer patients: a systematic review. Asia-Pacific Journal of Clinical Oncology 2018;14(6):374-82. - PubMed
NCCIH 2016
-
- National Centre for Complementary and Integrative Health (NCCIH). Complementary, alternative, or integrative health: what’s In a name? nccih.nih.gov/health/integrative-health (accessed prior to 10 September 2018).
NCI 2021
-
- National Cancer Institute. Drug dictionary: Blue Citrus-based herbal capsule. www.cancer.gov/publications/dictionaries/cancer-drug/def/blue-citrus-bas... (accessed 27 March 2021).
NICE 2014
-
- National Institute for Health and Care Excellence. Osteoarthritis: care and management. www.nice.org.uk/guidance/cg177 (accessed prior to 10 September 2018).
Niravath 2013
-
- Niravath P. Aromatase inhibitor-induced arthralgia: a review. Annals of Oncology 2013;24(6):1443-9. - PubMed
Niravath 2018
Olsen 2018
-
- Olsen MF, Bjerre E, Hansen MD, Tendal B, Hilden J, Hróbjartsson A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. Journal of Clinical Epidemiology 2018;101:87-106. - PubMed
OMERACT 2021
-
- Outcome Measures in Rheumatology. OMERACT Outcome Measurement. omeract.org/ (accessed 16 October 2021).
Partridge 2008
-
- Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. Journal of Clinical Oncology 2008;26(4):556-62. - PubMed
Patrick 2007
-
- Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value in Health 2007;10(Suppl 2):S125-37. - PubMed
Peng 2014
Poole 2013
Power 2004
-
- Power R. Emu oil for osteoarthritic hand pain [Masters thesis]. Melbourne (Australia): Victoria University, 2004. [URL: vuir.vu.edu.au/id/eprint/869]
Presant 2007
-
- Presant CA, Bosserman L, Young T, Vakil M, Horns R, Upadhyaya G, et al. Aromatase inhibitor-associated arthralgia and/ or bone pain: frequency and characterization in non-clinical trial patients. Clinical Breast Cancer 2007;7(10):775-8. - PubMed
Rastelli 2011
-
- Rastelli AL, Taylor ME, Gao F, Armamento-Villareal R, Jamalabadi-Majidi S, Napoli N, et al. Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): a phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Research and Treatment 2011;129(1):107-16. - PubMed
Reginster 2012
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Review Manager Web 2021 [Computer program]
-
- Review Manager Web (RevMan Web). Version 2.5.1. The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Richards 2012
Roberts 2017
-
- Roberts K, Rickett K, Greer R, Woodward N. Management of aromatase inhibitor induced musculoskeletal symptoms in postmenopausal early breast cancer: a systematic review and meta-analysis. Critical Reviews in Oncology/Hematology 2017;111:66-80. - PubMed
Roberts 2018
-
- Roberts KE, Rickett K, Chatfield MD, Woodward NE. Systemic therapies for preventing or treating aromatase inhibitor‐induced musculoskeletal symptoms in early breast cancer. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD013167. [DOI: 10.1002/14651858.CD013167] - DOI - PMC - PubMed
Roberts 2020
-
- Roberts KE, Rickett K, Feng S, Vagenas D, Woodward NE. Exercise therapies for preventing or treating aromatase inhibitor‐induced musculoskeletal symptoms in early breast cancer. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD012988. [DOI: 10.1002/14651858.CD012988.pub2] - DOI - PMC - PubMed
Rocque 2018
-
- Rocque G. What is the role of symptom management and patient-reported outcomes in adherence to aromatase inhibitors? Journal of Clinical Oncology 2018;36(4):308-9. - PubMed
Rosati 2011
-
- Rosati MS, Di Sri M, Baciarello G, Russo VL, Grassi P, Marchetti L. Etoricoxib and anastrozole in adjuvant early breast cancer: ETAN trial (phase III). Journal of Clinical Oncology 2011;29(15 Suppl):533.
Schmidt 2006
-
- Schmidt M, Naumann H, Weidler C, Schellenberg M, Anders S, Straub RH. Inflammation and sex hormone metabolism. Annals of the New York Academy of Sciences 2006;1069:236-46. - PubMed
Schünemann 2021
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook 2019:375-402. [DOI: 10.1002/9781119536604.ch14] - DOI
Sestak 2008
-
- Sestak I, Cuzick J, Sapunar F, Eastell R, Forbes JF, Bianco AR, et al. Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncology 2008;9(9):866-72. - PubMed
Shen 2018
Singh 2015
Smith 2013
Song 2016
-
- Song GG, Seo YH, Kim J, Choi SJ, Ji JD, Lee YH. Relative efficacy and tolerability of etoricoxib, celecoxib, and naproxen in the treatment of osteoarthritis: a Bayesian network meta-analysis of randomized controlled trials based on patient withdrawal. Zeitschrift fur Rheumatologie 2016;75(5):508-16. - PubMed
Sundrarjun 2004
-
- Sundrarjun T, Komindr S, Archararit N, Dahlan W, Puchaiwatananon O, Angthararak S, et al. Effects of n-3 fatty acids on serum interleukin-6, tumour necrosis factor-alpha and soluble tumour necrosis factor receptor p55 in active rheumatoid arthritis. Journal of International Medical Research 2004;32(5):443-54. - PubMed
Towheed 2005
Turner 2015
-
- Turner A, Hancock G, Wells J, Whitehouse M. Traditional medicinal oils sourced from birds: anti-inflammatories and potential immunoregulants. Progress in Drug Research 2015;70:155-78. - PubMed
Wan 2013
Whitehouse 1998
-
- Whitehouse MW, Turner AG, Davis CK, Roberts MS. Emu oil(s): a source of non-toxic transdermal anti-inflammatory agents in Aboriginal medicine. Inflammopharmacology 1998;6(1):1-8. - PubMed
WHO 2013
-
- World Health Organization. WHO traditional medicine strategy: 2014–2023. apps.who.int/iris/bitstream/handle/10665/92455/9789241506090_eng.pdf?seq... (accessed prior to 18 December 2021).
Wieland 2011
Williams 2011
-
- Williams DA, Arnold LM. Measures applied to the assessment of fibromyalgia: Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), the Multidimensional Fatigue Inventory (MFI-20), the MOS Sleep Scale, and the Multiple Ability Self-Report Questionnaire (MASQ; cognitive dysfunction). Arthritis Care & Research 2011;63(0 11):S86. - PMC - PubMed
Xian 2011
Xu 2006
-
- Xu W, Towers AD, Li P, Collet JP. Traditional Chinese medicine in cancer care: perspectives and experiences of patients and professionals in China. European Journal of Cancer Care 2006;15(4):397-403. - PubMed
Xu 2014
-
- Xu L, Wang J, Xue DD, He W. Aromatase inhibitors associated musculoskeletal disorders and bone fractures in postmenopausal breast cancer patients: a result from Chinese population. Medical Oncology 2014;31(9):128. - PubMed
Yang 2017
-
- Yang GS, Kim HJ, Griffith KA, Zhu S, Dorsey SG, Renn CL. Interventions for the treatment of aromatase inhibitor-associated arthralgia in breast cancer survivors: a systematic review and meta-analysis. Cancer Nursing 2017;40(4):E26-41. - PubMed
Yost 2014
Zhang 2010
-
- Zhang Q, Tang D, Zhao H. Immunological therapies can relieve aromatase inhibitor-related joint symptoms in breast cancer survivors. American Journal of Clinical Oncology 2010;33(6):557-60. - PubMed
References to other published versions of this review
Roberts
-
- Roberts KE, Rickett K, Chatfield MD, Woodward NE. Systemic therapies for preventing or treating aromatase inhibitor‐induced musculoskeletal symptoms in early breast cancer. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD013167. [DOI: 10.1002/14651858.CD013167] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

