Pharmacological inhibition of the lipid phosphatase PTEN ameliorates heart damage and adipose tissue inflammation in stressed rats with metabolic syndrome
- PMID: 35005845
- PMCID: PMC8744130
- DOI: 10.14814/phy2.15165
Pharmacological inhibition of the lipid phosphatase PTEN ameliorates heart damage and adipose tissue inflammation in stressed rats with metabolic syndrome
Abstract
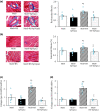
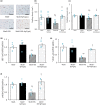
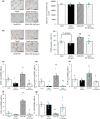
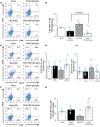
Phosphatidylinositol 3-kinase (PI3K) signaling promotes the differentiation and proliferation of regulatory B (Breg) cells, and the lipid phosphatase phosphatase and tensin homolog deleted on chromosome 10 (PTEN) antagonizes the PI3K-Akt signaling pathway. We previously demonstrated that cardiac Akt activity is increased and that restraint stress exacerbates hypertension and both heart and adipose tissue (AT) inflammation in DS/obese rats, an animal model of metabolic syndrome (MetS). We here examined the effects of restraint stress and pharmacological inhibition of PTEN on heart and AT pathology in such rats. Nine-week-old animals were treated with the PTEN inhibitor bisperoxovanadium-pic [bpV(pic)] or vehicle in the absence or presence of restraint stress for 4 weeks. BpV(pic) treatment had no effect on body weight or fat mass but attenuated hypertension in DS/obese rats subjected to restraint stress. BpV(pic) ameliorated left ventricular (LV) inflammation, fibrosis, and diastolic dysfunction as well as AT inflammation in the stressed rats. Restraint stress reduced myocardial capillary density, and this effect was prevented by bpV(pic). In addition, bpV(pic) increased the proportions of Breg and B-1 cells as well as reduced those of CD8+ T and B-2 cells in AT of stressed rats. Our results indicate that inhibition of PTEN by bpV(pic) alleviated heart and AT inflammation in stressed rats with MetS. These positive effects of bpV(pic) are likely due, at least in part, to a reduction in blood pressure, an increase in myocardial capillary formation, and an altered distribution of immune cells in fat tissue that result from the activation of PI3K-Akt signaling.
Keywords: PTEN; adipose tissue inflammation; cardiac injury; metabolic syndrome; regulatory B cell; stress.
© 2022 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Figures


References
-
- Baumgarth, N. (2011). The double life of a B‐1 cell: Self‐reactivity selects for protective effector functions. Nature Reviews Immunology, 11, 34–46. - PubMed
-
- Francis, W. R. , Ireland, R. E. , Spear, A. M. , Jenner, D. , Watts, S. A. , Kirkman, E. , & Pallister, I. (2019). Flow cytometric analysis of hematopoietic populations in rat bone marrow. Impact of trauma and hemorrhagic shock. Cytometry Part A, 95(11), 1167–1177. 10.1002/cyto.a.23903 - DOI - PMC - PubMed
-
- Harmon, D. B. , Srikakulapu, P. , Kaplan, J. L. , Oldham, S. N. , McSkimming, C. , Garmey, J. C. , Perry, H. M. , Kirby, J. L. , Prohaska, T. A. , Gonen, A. , Hallowell, P. , Schirmer, B. , Tsimikas, S. , Taylor, A. M. , Witztum, J. L. , & McNamara, C. A. (2016). Protective role for B‐1b B cells and IgM in obesity‐associated inflammation, glucose intolerance, and insulin resistance. Arteriosclerosis, Thrombosis, and Vascular Biology, 36, 682–691. 10.1161/ATVBAHA.116.307166 - DOI - PMC - PubMed
-
- Hattori, T. , Murase, T. , Takatsu, M. , Nagasawa, K. , Matsuura, N. , Watanabe, S. , Murohara, T. , & Nagata, K. (2014). Dietary salt restriction improves cardiac and adipose tissue pathology independently of obesity in a rat model of metabolic syndrome. Journal of the American Heart Association, 3, e001312. 10.1161/JAHA.114.001312 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials

