Controlled Modular Multivalent Presentation of the CD40 Ligand on P22 Virus-like Particles Leads to Tunable Amplification of CD40 Signaling
- PMID: 35005938
- PMCID: PMC9136706
- DOI: 10.1021/acsabm.1c00718
Controlled Modular Multivalent Presentation of the CD40 Ligand on P22 Virus-like Particles Leads to Tunable Amplification of CD40 Signaling
Abstract
Ligands of the tumor necrosis factor superfamily (TNFSF) are appealing targets for immunotherapy research due to their integral involvement in stimulation or restriction of immune responses. TNFSF-targeted therapies are currently being developed to combat immunologically based diseases and cancer. A crucial determinant of effective TNFSF receptor binding and signaling is the trimeric quaternary structure of the ligand. Additionally, ligand multivalency is essential to propagate strong signaling in effector cells. Thus, designing a synthetic platform to display trimeric TNFSF ligands in a multivalent manner is necessary to further the understanding of ligand-receptor interactions. Viral nanocages have architectures that are amenable to genetic and chemical modifications of both their interior and exterior surfaces. Notably, the exterior surface of virus-like particles can be utilized as a platform for the modular multivalent presentation of target proteins. In this study, we build on previous efforts exploring the bacteriophage P22 virus-like particle for the exterior multivalent modular display of a potent immune-stimulating TNFSF protein, CD40 ligand (CD40L). Using a cell-based reporter system, we quantify the effects of tunable avidity on CD40 signaling by CD40L displayed on the surface of P22 nanocages. Multivalent presentation of CD40L resulted in a 53.6-fold decrease of the half maximal effective concentration (EC50) compared to free CD40L, indicating higher potency. Our results emphasize the power of using P22-based biomimetics to study ligand-receptor interactions within their proper structural context, which may contribute to the development of effective immune modulators.
Keywords: CD40; CD40L; P22; TNFSF; VLP; biomimetics; multivalency; signaling.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

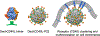

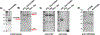

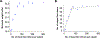
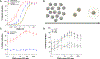
References
-
- Mammen M; Choi SK; Whitesides GM Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem., Int. Ed. 1998, 37, 2754–2794. - PubMed
-
- Kwa S; Lai L; Gangadhara S; Siddiqui M; Pillai VB; Labranche C; Yu T; Moss B; Montefiori DC; Robinson HL; Kozlowski PA; Amara RR CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge. J. Virol. 2014, 88, 9579–9589. - PMC - PubMed
-
- Tansey MG; Szymkowski DE The TNF superfamily in 2009: new pathways, new indications, and new drugs. Drug Discovery Today 2009, 14, 1082–1088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
